Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Radioactive Decay
Some materials are radioactive. They emit radiation. When an atom of a radioactive substance emits radiation, it is transformed to a new type of atom. This process is called radioactive decay.
There are many types of radiation that can be emitted during radioactive decay. Particle radiation includes alpha and beta particles as well as proton and neutron radiation. Electromagnetic radiation includes high energy gamma rays and X-rays.
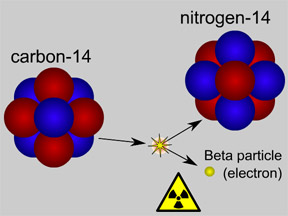
Most elements come in various "versions", called isotopes, with different numbers of neutrons and slightly differing properties. In many cases, less-common isotopes of common substances are radioactive. For example, the rare isotope of carbon called carbon-14 is radioactive. It has 8 neutrons (instead of the usual 6) and radiates beta particles. When an atom emits radiation and undergoes radioactive decay, it may be transformed from one isotope to another, or it may become a different element altogether. When carbon-14 decays by emitting a beta particle, it becomes nitrogen-14. Isotopes that do not decay are said to be "stable".
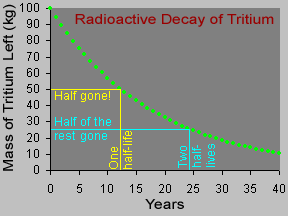
Different radioactive materials take different amounts of time to decay. Scientists use the idea of a half-life to express this. For example, tritium is a radioactive isotope of hydrogen with a half-life of 12.3 years. If we started with 100 kg of radioactive tritium, 12.3 years later we would have just half that - 50 kg! The other half would have become helium-3 via radioactive decay. During the 12.3 years after that, half of the remaining 50 kg of tritium would decay, leaving just 25 kg of tritium. After two half-lives, only one-quarter (half of half) of the original radioactive substance would remain - 25 kg out of the original 100 kg. The half-life of a radioactive material can be very short (less than a second) or very long (thousands of years) or anywhere in between.