Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Isotope
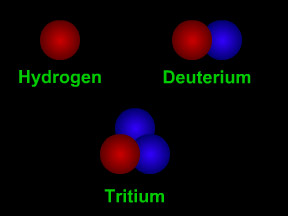
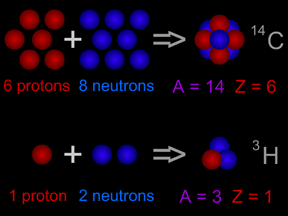
Isotopes are different "versions" of a chemical element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six protons, and all uranium atoms have 92 protons. However, the number of neutrons in atoms of an element can vary. For instance, while most carbon atoms have six neutrons, about 1% of the carbon atoms found on Earth have seven neutrons and an even smaller fraction of carbon atoms have eight neutrons. All carbon atoms have an atomic number (number of protons) of six, but the different isotopes of carbon can have an atomic mass (number of protons + neutrons) of 12, 13 or 14.
Two types of notation are commonly used to indicate isotopes. As an example, carbon's 8-neutron isotope is written as either carbon-14 or 14C. For the most part, all of the isotopes of a particular element behave the same way in chemical reactions. For instance, an atom of the rare oxygen-18 isotope will combine just as readily with two hydrogen atoms to form water (H2O) as will a more common oxygen-16 atom.
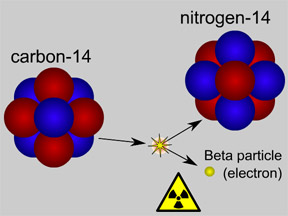
Some isotopes are radioactive, while others are not. Atoms of radioactive isotopes can "decay" by emitting energy in the form of some type of radiation. Atoms that decay typically lose one or more proton(s) and/or neutron(s), converting the atom to a different isotope or even a different element. Isotopes that are not radioactive are called "stable" isotopes. Of the 94 elements that occur naturally on Earth, 80 have at least one stable isotope. There are 26 elements that have only one stable isotope. Tin has the most, with ten stable isotopes. Of the 80 elements with at least one stable isotope, the average number of stable isotopes is 3.2 per element.
Tables that list the atomic masses of elements factor in the relative abundance of each isotope. For example, the atomic mass of chlorine is reported as 35.5 since 76% of chlorine found on Earth is the lighter 35Cl while 24% is the heavier 37Cl. Where do these different isotopes come from, and why are there differing quantities of each in nature? Astronomers believe that the only elements created in the Big Bang were various isotopes of hydrogen, helium, and probably lithium, beryllium and boron. Since then, enormous supernova explosions have created the remaining elements, including most of their isotopes. Some isotopes form when high-energy cosmic rays collide with various atoms. Isotopes are also created when other radioactive isotopes decay.
Isotopes often give scientists clues about the history of natural processes. Carbon dating techniques help us determine the age of objects based on the relative abundance of stable 12C as compared to radioactive 14C (which decays and "disappears" over time). The ratio of deuterium to normal hydrogen atoms in water molecules in ice core samples helps us estimate global temperatures in the past; the slightly heavier water molecules that contain a deuterium atom evaporate from the oceans (and later snow out over the poles) at a different rate as compared to their normal hydrogen cousins, hinting at average temperatures.