Click on image for full size
Original artwork by Windows to the Universe staff.
Radioactive
Some materials give off radiation. We say that those substances are "radioactive". Radioactive materials are often, though not always, hazardous to living things.
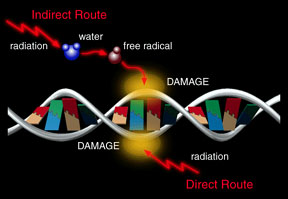
There are many different types of radioactive materials. Some emit particle radiation, like alpha or beta particles or neutron radiation. Some give off electromagnetic radiation, such as gamma rays or X-rays.
Most elements come in various "versions", called isotopes, with different numbers of neutrons and slightly differing properties. In many cases, less-common isotopes of common substances are radioactive. For example, the rare isotope of carbon called carbon-14 is radioactive. It has 8 neutrons (instead of the usual 6) and radiates beta particles. When they emit radiation, radioactive substances undergo radioactive decay. The element may be transformed from one isotope to another, or may become a different element altogether. When carbon-14 decays by emitting a beta particle, it becomes nitrogen-14. Isotopes that do not decay are said to be "stable".
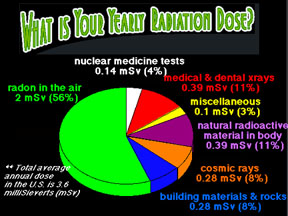
Radioactive isotopes can be dangerous to living things and damaging to equipment such as electronics. The level of danger depends, however, on the amount of radioactive substance present and the type of radiation and the rate of decay. Radioactive materials exist (mostly in trace quantities) in nature all around us. Small enough doses of most types of radiation cause us little harm.
Humans make use of radioactive materials in many ways. We use them in medicine to "label" and detect certain substances or tissues. We use them to determine the ages of artifacts via radiocarbon (carbon-14) dating. We also use them for nuclear power and in nuclear weapons.