Radioactive decay of an atom of carbon-14 yields a nitrogen-14 atom. A beta particle of radiation is emitted during the decay process.
Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Carbon-14 Dating (Radiocarbon Dating)
Carbon-14 dating (also called "radiocarbon dating") is used to determine the age of materials that contain carbon that was originally in living things. It is often used in archeology and some types of biology. Living creatures ingest carbon. Plants (and other autotrophs) take in carbon dioxide gas from the atmosphere during photosynthesis. Animals (and other heterotrophs) get their carbon by eating plants or other animals, from decaying organic matter, or from other similar sources.
Some of the carbon is a radioactive isotope called carbon-14 (14C). When the creature dies, it stops ingesting carbon. The radioactive 14C gradually undergoes radioactive decay, transforming it into nitrogen, and therefore gradually "disappears". Scientists can study samples from the once-live creatures' remains to see how much radioactive 14C, as compared to the normal isotope of carbon (carbon-12 or 12C), is still around. This tells the scientists how long ago the organism died.
14C has a half-life of 5,730 years, meaning that after about five thousand years about half of the 14C will decay and turn into nitrogen. After several half-lives, too little 14C will remain in a sample for it to be useful for dating. Radiocarbon dating is therefore only useful for samples with ages of less than about 65,000 to 80,000 years.
You might also be interested in:

Autotrophs are organisms that produce organic compounds from an inorganic source of carbon (carbon dioxide) given a source of energy. If the source of energy is the reactions of inorganic chemical compounds,
...more
Photosynthesis is the name of the process by which autotrophs (self-feeders) convert water, carbon dioxide, and solar energy into sugars and oxygen. It is a complex chemical process by which plants and
...more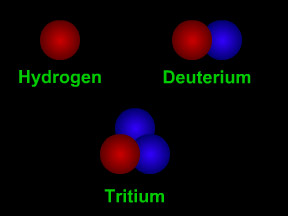
Isotopes are different "versions" of a chemical element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six protons, and
...more
Carbon-14 is an isotope of the element carbon. All carbon atoms have 6 protons in their nucleus. Most carbon atoms also have 6 neutrons, giving them an atomic mass of 12 ( = 6 protons + 6 neutrons). Carbon-14
...more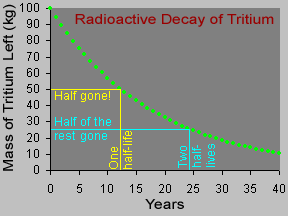
Physicists use the term "half-life" to describe how long it takes for radioactive materials to decay. When an atom of a radioactive substance decays, it emits radiation and changes into a different type
...more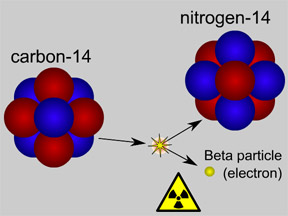
Some materials are radioactive. They emit radiation. When an atom of a radioactive substance emits radiation, it is transformed to a new type of atom. This process is called radioactive decay. There are
...more
Nitrogen is a chemical element with an atomic number of 7 (it has seven protons in its nucleus). Molecular nitrogen (N2) is a very common chemical compound in which two nitrogen atoms are tightly bound
...more