This image is a model of an atom
Windows to the Universe original animation by Randy Russell.
Related links:
Elements (Chemical Elements)
Atomic Nucleus
What's a molecule?
A Matter of Scale - interactive showing the sizes of things, from very tiny to huge - from NSF
Atoms
Atoms are the smallest size pieces that elements come in. Each atom has a central nucleus surrounded by a cloud of electrons. The nucleus contains one or more protons and usually about as many neutrons as protons. A neutral (electrically) atom has just as many (negatively charged) electrons as (positively charged) protons.
The animation shown here is NOT to scale. The distance between the nucleus and the electrons in an actual atom are much larger in comparison with the sizes of the protons, neutrons, and electrons. Most of an atom is empty space. Atoms are incredibly small, ranging in size from about 60 to 500 picometers (a picometer is 10-12 meters, or one trillionth of a meter). The nucleus of an atom is typically about 100,000 times smaller than the atom as a whole. Atoms have masses on the order of 10-27 to 10-25 kilograms. Almost all of an atom's mass (>99%) is in the nucleus; protons and neutrons are much more massive than electrons.
Sometimes two or more atoms "stick together" to form a molecule. Some molecules have atoms of just one type; for example, an oxygen molecule (O2) has two oxygen atoms. Other molecules combine different types of atoms. Methane (CH4) has one carbon atom and four hydrogen atoms.
You might also be interested in:

An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more
"Atomic mass" is a term physicists use to describe the size (mass) of an atom of a specific type. Since the nucleus of an atom contains nearly all (more than 99%) of an atom's mass, "atomic mass" is more-or-less
...more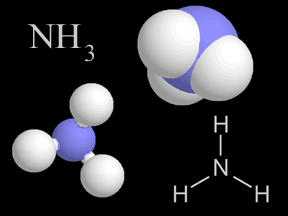
Most things around us are made of groups of atoms bonded together into packages called molecules. The atoms in a molecule are held together because they share or exchange electrons. Molecules are made
...more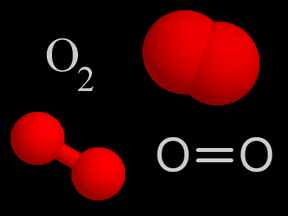
Oxygen is a chemical element with an atomic number of 8 (it has eight protons in its nucleus). Oxygen forms a chemical compound (O2) of two atoms which is a colorless gas at normal temperatures and pressures.
...more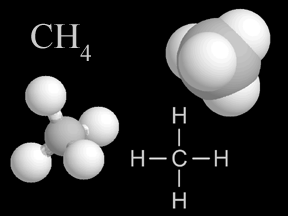
Methane is gas that is found in small quantities in Earth's atmosphere. Methane is the simplest hydrocarbon, consisting of one carbon atom and four hydrogen atoms. Methane is a powerful greenhouse gas.
...more
The atomic number of an atom tells us how many protons are in the nucleus of that atom. Why is that important? The chemical properties of an element are determined by the number of electrons in its atoms,
...more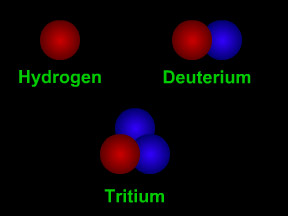
Isotopes are different "versions" of a chemical element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six protons, and
...more













