Click on image for full size
Windows to the Universe original artwork by Randy Russell.
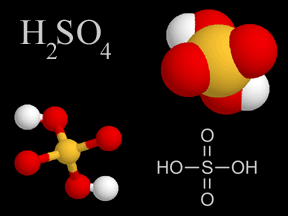
Sulfuric Acid - H2SO4
Sulfuric acid is a viscous, oily liquid and a strong acid which can cause severe burns. Sulfuric acid consists of sulfur, oxygen, and hydrogen atoms.
Sulfuric acid is one of the components of acid rain. Some types of pollution, especially the gases formed by the combustion of sulfur-rich coal and oil, contain sulfur dioxide (SO2). When mixed with droplets of water in Earth's atmosphere, sulfur dioxide gas changes to sulfur trioxide (SO3) and then to sulfuric acid. These droplets containing sulfuric acid fall from the sky as acid rain.
Humans produce more than 100 million tons of sulfuric acid worldwide each year. In fact, water is the only chemical compound we produce more of! Sulfuric acid is used in the production of phosphate fertilizers, for processing ores and wastewater, in the refining of oil and other petroleum products, and in the manufacture of some dyes. Lead-acid batteries (such as car batteries) and drain cleaners contain sulfuric acid.
Both natural and human sources release millions of tons of sulfur dioxide gas into Earth's atmosphere each year. Volcanoes are nature's main sulfur dioxide polluters. The oceans, biological decay, and forest fires are other natural sources of SO2. About three-fourths of the sulfur dioxide humans release comes from burning fossil fuels; half comes from burning coal, and 25-30% comes from burning oil. Other human-generated sources include smelting, the manufacture of sulphuric acid, conversion of wood pulp to paper, and the incineration of refuse. As mentioned above, some of the sulfur dioxide in the atmosphere is converted into sulfuric acid upon contact with water, and is thus a major source of acid rain.
The hot atmosphere of the planet Venus contains sulfuric acid, making it difficult to build spacecraft that can withstand the harsh Venusian atmosphere.
This well-known ditty warns of the dangers of consuming sulfuric acid:
- Little Johnny took a drink, but he shall drink no more.
- For what he thought was H2O, was H2SO4.