Photo courtesy of Nancy A. Marley
Related links:
Smog City Simulator (outside link)
Listen to a Podcast about How Divorce is Bad for the Environment
NSF News: Finding Answers in the Clouds
News from NSF: Scientists to Assess Beijing Olympics Air Pollution Control Efforts (08/07/08)
NSF News: Can Dying Trees Change Weather and Climate?
Our Changing Planet: Twelve Key Indicators of Climate Change (Videos and Classroom Activities)
Air Pollution
What do smog, acid rain, carbon monoxide, fossil fuel exhausts, and tropospheric ozone have in common? They are all examples of air pollution. Air pollution is not new. As far back as the 13 th century, people started complaining about coal dust and soot in the air over London, England. Since the beginning of the industrial revolution in the late 18 th century, humanity has been modifying the Earth's atmosphere and its chemistry. As industry spread across the globe, so did air pollution. Air pollution has many effects. In addition to being ugly, it can cause illness and even death, as well as damaging buildings, crops, and wildlife. Perhaps the worst air pollution recorded happened when dense smog (the combination of smoke and fog) formed over London on December 4, 1952 and lasted until March of 1953. Within six days over 4,000 people died, and 8,000 more died within six months.
Air pollution is a broad term that is applied to particulate matter and chemical compounds that are released by humans into the atmosphere and modify its composition. It was first perceived as a local problem in urban industrialized areas, so factories and power plants started building taller smoke-stacks. However, taller stacks merely transported the problem elsewhere and soon regional problems such as acid rain were recognized. In Scandanavia, for example, the acidification of lakes was found to be the result of sulfur dioxide emissions from tall stacks located in central European countries such as Germany and even in places as far off as Great Britain. More recently, global problems such as climate change and stratospheric ozone depletion have been widely publicized.
Natural sources that affect atmospheric chemistry include sulfur and nitrogen compounds from volcanoes and biological decay and particulate matter from dust storms and volcanoes. Plants, trees, and even grasses release volatile organic compounds (VOCs), such as methane, into the air. Of more concern, since we have the ability to control them, are anthropogenic, or man-made, air pollutants, such as carbon monoxide, sulfur dioxide, some fraction of VOCs, and nitrogen oxides. The largest source of anthropogenic pollution is the burning of fossil fuels, including coal, oil, and gas, in our homes, factories, and vehicles.
Air pollutants are classified as either primary or secondary. A primary pollutant is one that is released directly to the air, such as the carbon monoxide from combustion. A secondary pollutant is formed in the atmosphere through chemical reactions of primary pollutants. The formation of tropospheric ozone is an example of secondary air pollution.
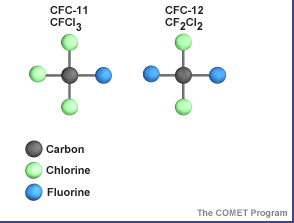
The atmosphere is a complex, dynamic and fragile system. Concern is growing about the global effects of air pollutant emissions, especially health effects, climate change, and the effects of pollution on crops. Stratospheric ozone depletion due to air pollution has long been recognized as a threat to human health.