Click on image for full size
JPL
Sublimation
Sublimation is an unusual change of state. We are all familiar with evaporation, the change of state that occurs when a liquid turn into a gas. On a hot day, a puddle of water gradually "disappears" as the liquid water evaporates and becomes water vapor, as gas. Likewise, everyone knows about melting, the change of state from a solid to a liquid. Ice, the solid state of water, will melt and become liquid water if the temperature is above 0° C (32° F). Most people are not, however, familiar with sublimation. Under certain conditions, a solid material can change directly into a gas... without first becoming a liquid. This change of state directly from a solid to a gas is called sublimation.
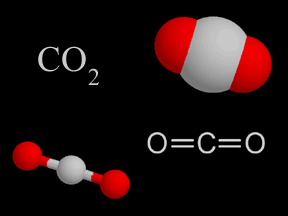
Perhaps you've seen "dry ice" sublimate. Dry ice (frozen carbon dioxide) can sublimate at temperatures and pressures commonly found on Earth. Various other substances can also sublimate, depending on the temperature and pressure. Sublimation is more common at low pressures; lower than the pressures typically found in the part of Earth's atmosphere where we live. The opposite of sublimation is deposition, when a gas turns directly into a solid (just as condensation is the opposite of evaporation and freezing is the opposite of melting). The formation of frost is an example of deposition.
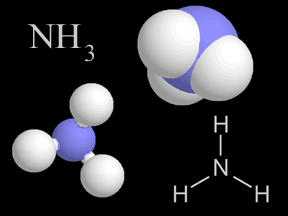
Sublimation is common on the surfaces of many objects in our solar system, including some planets, many of the icy moons, and comets. Many of these objects either have no atmosphere or a very thin (low pressure) atmosphere. Ices made of ammonia, carbon dioxide, and water are common on the surfaces of these "worlds"; all of these ices can sublimate when heated if the pressure is low. For example, the polar ice caps of Mars include lots of "dry ice" which sublimates into the Martian atmosphere during the summer season at each pole.
Comets are spectacular examples of sublimation. Comets are mostly made of ices. As a comet approaches the Sun, the sunlight warms and sublimates huge amounts of ice. The released gases form a hazy "cloud" or temporary atmosphere (called the "coma") around the comet. The weak gravity of a comet cannot hold onto this temporary atmosphere. As the coma is swept away from the comet, it forms the spectacular tails that make comets such a delight to observe.