Sulfur oxides - Sulfur dioxide (SO2) and Sulfur trioxide (SO3)
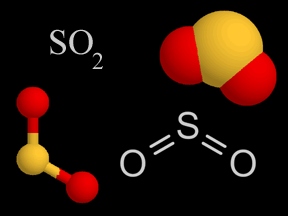
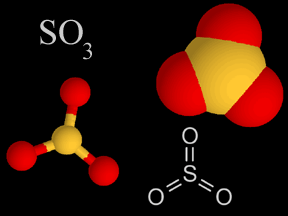
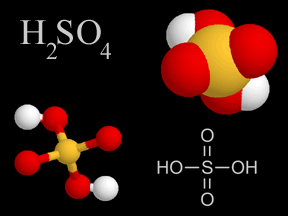
Sulfur dioxide (SO2) and sulfur trioxide (SO3) are two chemical compounds whose molecules are made up of one sulfur atom and multiple oxygen atoms. Know together as sulfur oxides, these substances are important atmospheric pollutants. Sulfur dioxide (SO2) is a colorless, dense, toxic, nonflammable gas with a strong odor. At normal temperatures and pressures, sulfur trioxide is a liquid. Oxygen-rich sulfur trioxide (SO3) is highly reactive, and readily combines with many substances. Sulfur oxides are present naturally at low concentrations in Earth's atmosphere, and at much higher concentrations in polluted urban environments. Natural sources include volcanoes, the oceans, biological decay, and forest fires. Fossil fuel combustion accounts for 75 to 80% of human production of sulfur oxides in the air. Coal burning generates about half of the sulfur oxide emissions we release, while burning oil accounts for 25 to 30%. Smelting, manufacture of sulfuric acid, conversion of wood pulp to paper, and incineration of refuse are other anthropogenic sources of atmospheric sulfur oxides. Sulfur dioxide is itself a pollutant which causes respiratory problems; it is especially irritating to the lungs. Sulfur oxides are a major culprit in the production of acid rain. Sulfur dioxide oxidizes via several chemical pathways to sulfur trioxide. Sulfur trioxide then combines with water vapor or droplets to make sulfuric acid (H2SO4). Sulfuric acid is one of the types of acid in acid rain. Scientists estimate that natural sources release 80 to 290 million tons of sulfur oxides into Earth's atmosphere each year. Humans contribute another 70 to 100 tons annually. The United States releases about 20 million tons of sulfur dioxide into the air each year. For comparison, the large eruption of the volcanic Mt. Pinatubo in the Philippines in 1991 released between 15 and 30 million tons. Most fossil fuels have some sulfur in them. When the fuel is burned, some of the sulfur combines with oxygen to generate sulfur oxides. Different types and sources of fossil fuels have different levels of sulfur contaminants. Oil from the Middle East has a low sulfur content, whereas oil from Venezuelan, for example, has more sulfur in it. Low sulfur oil burns cleaner, and is thus in higher demand and more expensive. In the U.S., most coal mined east of the Mississippi River has a relatively high sulfur content, while much of the coal from western states has less sulfur. The concentration of sulfur dioxide in clean air is around 0.01 ppm (parts per million) or less. In a polluted urban environment, the concentration rises to between 0.1 and 2 ppm, a ten- to two hundred-fold increase. Sulfur oxides do have some beneficial uses. Sulfur dioxide is employed as a preservative in certain alcoholic drinks and in some foods, such as dried fruits. It is also used to suppress growth of wild yeasts and bacteria during winemaking. Large quantities of sulfur dioxide are created as a feedstock in the production of sulfuric acid, one of the most widely used chemical substances in industry. |