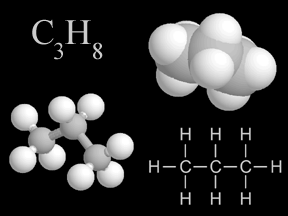
Shown here are four representations chemists use for propane, a common hydrocarbon. In the colored models, carbon is light gray and hydrogen is white.
Click on image for full size
Windows to the Universe original artwork by Randy Russell.
Hydrocarbons
There is a large class of important chemical compounds whose molecules are made up entirely of carbon and hydrogen atoms. These compounds, as a group, are called "hydrocarbons".
Hydrocarbons are the critical energy storage molecules within all major types of fossil fuels (including coal, oil, and natural gas) and biofuels. They also form the feedstock for the production processes of many types of plastics.
Burning hydrocarbons in the presence of oxygen (O2) produces carbon dioxide (CO2) and water (H2O). If there is too much carbon or too little oxygen present when hydrocarbons are burned, carbon monoxide (CO) may also be emitted. Sometimes unburned hydrocarbons are released into the air during incomplete combustion.
Burning fossil fuels, including gasoline in automobile engines, releases some hydrocarbons into the air. In a typical urban environment, the atmospheric concentration of hydrocarbons is around 3 ppm (parts per million). Some hydrocarbons, along with other types of Volatile Organic Compounds (VOCs), contribute to the formation of photochemical smog.
The carbon atoms in hydrocarbons often form long chains or ring structures. Some of the hydrocarbons that you may have heard of include methane (CH4), butane (C4H10), propane (C3H8), benzene (C6H6), ethane (C2H6), and hexane (C6H14).
You might also be interested in:
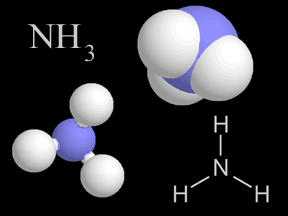
Most things around us are made of groups of atoms bonded together into packages called molecules. The atoms in a molecule are held together because they share or exchange electrons. Molecules are made
...more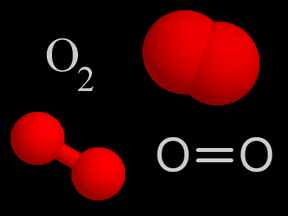
Oxygen is a chemical element with an atomic number of 8 (it has eight protons in its nucleus). Oxygen forms a chemical compound (O2) of two atoms which is a colorless gas at normal temperatures and pressures.
...more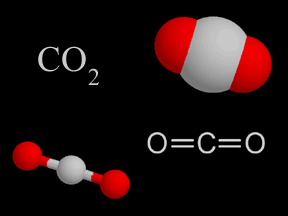
Carbon dioxide is a colorless and non-flammable gas at normal temperature and pressure. Although much less abundant than nitrogen and oxygen in Earth's atmosphere, carbon dioxide is an important constituent
...more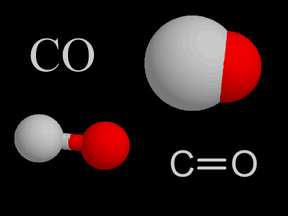
Carbon monoxide is a colorless, odorless, tasteless gas. It is also flammable and is quite toxic to humans and other oxygen-breathing organisms. A molecule of carbon monoxide (CO) contains one carbon atom
...more
What do smog, acid rain, carbon monoxide, fossil fuel exhausts, and tropospheric ozone have in common? They are all examples of air pollution. Air pollution is not new. As far back as the 13 th century,
...more
Air pollution comes from many different sources. Natural processes that affect air quality include volcanic activity, which produce sulfur, chlorine, and ash particulates, and wildfires, which produce
...more
Volatile Organic Compounds or VOCs are organic chemicals that easily vaporize at room temperature. They are called organic because they contain the element carbon in their molecular structures. VOCs include
...more