Click on image for full size
Credit: UNC
Acidic Ocean Water Impacts Corals and Other Marine Life
Some of the carbon dioxide that is added to the atmosphere from burning fossil fuels makes its way into the world’s oceans. This makes seawater more acidic, which could have a large impact on marine life.
Marine creatures such as corals, clams, snails, and many other types of marine life build their skeletons and shells from calcium carbonate. These creatures get the materials they need to make the calcium carbonate from seawater. As seawater gets more acidic these creatures might have a harder time building their skeletons and shells because calcium carbonate mineral dissolves in acid.
Put a clam shell (one that you don’t want to keep) into a container of vinegar and wait. Vinegar is an acid. Within a few hours will notice that your clam shell is disappearing. The calcium carbonate that makes up the shell is dissolving into the acidic vinegar.
Seawater will not become as acidic as vinegar. It has become only slightly more acidic over the past 150 years. It will continue to get more acidic, but very slowly. Scientists suspect that even a small change can make a big difference to the creatures that need to build their shells.
Because corals build reefs from calcium carbonate, and because those reefs become a home to a large amount of marine life, scientists are especially interested in the impact of more acidic water on corals.
Slower growing shells and skeletons can have an impact on the food webs of marine life, possibly changing the number of species of living things in the ocean.
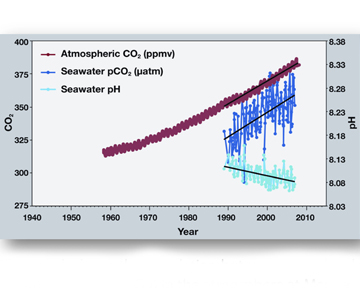
Sometimes a picture is worth a thousand words. This image shows the link between rising levels of carbon dioxide (CO2) in the atmosphere with rising CO2 levels in the nearby ocean (at Mauna Loa, Hawaii). As more CO2 builds up in the ocean, the pH of the ocean decreases (gets more acidic).