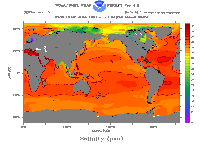
Click on image for full size
Image courtesy of NOAA
Salinity
About 70% of the Earth is covered with water, and we find 97% of that water in the oceans. Everyone who has taken in a mouthful of ocean water while swimming knows that the ocean is really salty.All water has some dissolved material in it. This dissolved material can be solids, liquids or gases that have completely mixed with the water. This dissolved material which can come from the land, precipitation, or the atmosphere, is also referred to as dissolved salts. Dissolved salts are found in river water, groundwater, rain water, lake water, drinking water and so on. Yet, these are all considered fresh water. The difference between fresh water and ocean water is that ocean water contains many more dissolved salts.
Ocean water is about 3.5% salt. That means that if the oceans dried up completely, enough salt would be left behind to build a 180-mile-tall, one- mile-thick wall around the equator. And more than 90 percent of that salt would be sodium chloride, or ordinary table salt.
The oceans sure contain a lot of salt. How did that salt come to be there? It is thought that when the Earth was still young, many of the gases emitted from volcanoes dissolved in the primitive ocean, originally making it salty. Now, one of the main contributors of salt in the ocean is the continual rinsing of the Earth. Rivers, streams and groundwater all flow into the oceans. They carry water and dissolved minerals or salts from the rocks they have washed over. These dissolved salts then get deposited in the ocean. Another great contributor of salts to the ocean comes from the mid-ocean ridges, the areas of sea floor spreading. So, the oceans are getting saltier every day...but the rate of increase is so small we can't even measure it yet!
From the top of the ocean all the way to the depths of the ocean, salinity is between ~33-37 ppt or psu (average salinity of the ocean is 35 ppt). You can see from the image shown on this page that salinity for almost the entire ocean (at sea surface) is colored some shade of orange, corresponding to a salinity measurement around 33-36 ppt or psu. As shown on this graph, there are some geographic variations of salinity that are of interest. Salinity of the top layer of the ocean is closely linked with precipitation and evaporation. Evaporation leaves behind dissolved salts increasing salinity and precipitation "freshens" the top ocean layers. So, salinity is high in mid-latitudes where evaporation is high and precipitation is low (darker oranges). Salinity is low near the equator because precipitation is so high (lighter orange). Very high latitudes can also see decreases in salinity where sea ice melts and "freshens" the water (light orange and even yellow in the Arctic).
The oceans are naturally salty. The saline environment has quite an effect on life in the oceans. Most creatures that live in the ocean could not live in fresh water. However, when the highly saline waters of the ocean meet fresh water, an estuary is formed. This is a special environment where some creatures have learned to adapt to a mixture of fresh and salt water. When fresh water, ground water and soils are altered by human actions and salinity greatly increases, it can have an extreme detrimental effect on life there.
Changes in salinity brought about by human residential, commercial and industrial activity can kill plant life, aquatic life, and animal life in a given area. Humans have the responsibility to make sure their actions are not causing this type of devastation. For more information on salinity and its effect on plants, soils and water, please use the links below...