Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Half-Life
Physicists use the term "half-life" to describe how long it takes for radioactive materials to decay. When an atom of a radioactive substance decays, it emits radiation and changes into a different type of atom (a different isotope or element). Different radioactive materials decay at different rates; some decay in a matter of seconds or minutes, while others take millions of years. A short "half-life" means the substance decays quickly; a long half-life indicates a slower rate of decay.
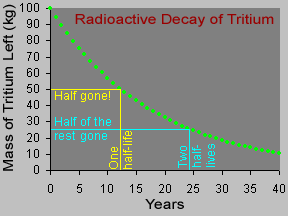
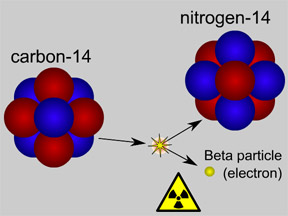
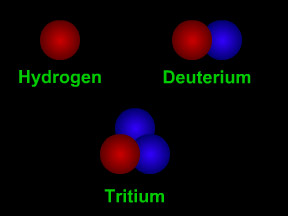
Not all atoms of a particular type of radioactive material will decay at the same time. However, for a sample with a large number of atoms the laws of probability allow us to say something about the average decay rate. For any substance, after a certain amount of time, about half of the atoms in the sample will have decayed. That "certain amount of time" is called the half-life of that particular substance. Different radioactive substances have different half-lives. For example, tritium, a radioactive isotope of hydrogen, has a half-life of 12.3 years; while carbon-14 has a half-life of nearly 6,000 years and uranium-235 has a half-life of more than 700 million years.
Let's say you had a sample of 100 kilograms of tritium. After 12.3 years (the half-life of tritium), half of the tritium would be "gone", having emitted beta particles and decayed to form helium-3. The other half of the tritium, 50 kg, would remain. After another 12.3 years (24.6 years from the start), half of the remaining 50 kg of tritium would decay, so only 25 kg of the original 100 kg would still be tritium. Another 12.3 years later (36.9 years from the start), half of the 25 kg of tritium remaining would decay, leaving us with one-eighth (12.5 kg) of the original 100 kg sample.
Knowing the half-life of a radioactive material can help us determine the age of an object. The amount of radioactive carbon-14 left in a once-living object (wood, bones or teeth, etc.) can be used to determine how long ago the creature died. Isotopes with short half-lives are often used in medicine; the fast decay rates of such isotopes prevent radiation from lingering in the body. The challenge of disposal of radioactive isotopes with long half-lives is a major problem associated with the use of nuclear power for electricity.