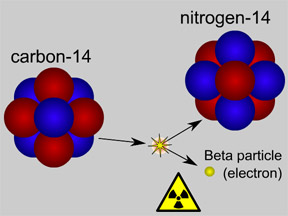
The atomic nucleus shown in the top half of this picture is carbon-14. The
14C nucleus has 6 protons plus 8 neutrons, giving it an atomic mass of 14.
Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Carbon-14
Carbon-14 is an isotope of the element carbon. All carbon atoms have 6 protons in their nucleus. Most carbon atoms also have 6 neutrons, giving them an atomic mass of 12 ( = 6 protons + 6 neutrons). Carbon-14 atoms have two extra neutrons, giving them a total of 8 neutrons. Carbon-14 has an atomic mass of 14 ( = 6 protons + 8 neutrons). The extra neutrons make the nucleus of carbon-14 unstable. Carbon-14 is radioactive!
Radioactive carbon-14 (also written as 14C) has a half-life of 5,730 years. 14C is used to determine the ages of artifacts that were once living (such as pieces of wood, teeth or bones, coral skeletons, etc.) via a technique called "carbon-14 dating" or "radiocarbon dating".
Some of the carbon dioxide gas in Earth's atmosphere contains 14C atoms. The supply of CO2 molecules which contain carbon-14 is continuously replenished in our atmosphere. Cosmic rays from space sporadically strike nitrogen atoms, converting some common nitrogen-14 atoms into radioactive carbon-14 atoms.
You might also be interested in:
Carbon-14 dating (also called "radiocarbon dating") is used to determine the age of materials that contain carbon that was originally in living things. It is often used in archeology and some
...more
An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more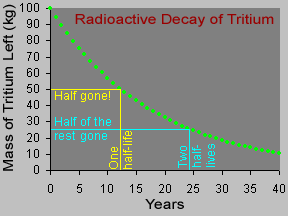
Some materials are radioactive. Their atoms give off radiation. When an atom gives off radiation, it turns into a different kind of atom. That is called radioactive decay. Some atoms decay very quickly,
...more
Some materials are radioactive. They give off radiation. When an atom of a radioactive substance gives off radiation, it becomes a new type of atom. This change is called radioactive decay. There are two
...more
Looking for online content that can be used for a climate change education course or module? Pages linked below can be used to support an introductory climate change education for either a unit or a full
...more
Did you know that a lot of what happens in space physics has to do with the interaction of really tiny particles? These particles like protons and electrons are so tiny that you can't even see them with
...more
Text for this level has not been written yet. Please see the "Intermediate" text for this page if you want to learn about this topic. To get to the "Intermediate" text, click on the blue "Intermediate"
...more














