Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
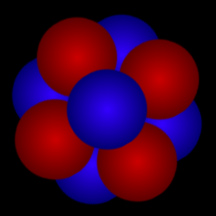
Atomic Nucleus
Atoms are composed of a massive, central nucleus surrounded by a swarm of fast-moving electrons. The nucleus is made up of protons and, in most cases, neutrons. Almost all of the mass (more than 99%) of an atom is contained in the dense nucleus.
An atomic nucleus is much, much smaller than an atom. The cloud of electrons that "orbit" the nucleus and define the "size" of an atom is roughly 100,000 times as large as that atom's nucleus! For example, a helium atom has a size of about 1 Ångström (0.1 nanometers or 10-10 meters), while its nucleus is only 1 femtometer (10-15 meters) in diameter. If you made a scale model of an atom with a nucleus the size of a pea, the electrons would zing around in a space larger than a major sports stadium! An atom is mostly empty space.
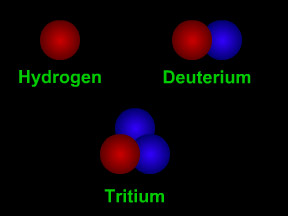
The number of protons in the nucleus determines what type of element the atom is. The number of protons is called the element's "atomic number". For example, hydrogen has an atomic number of one, since all hydrogen atoms have one proton in their nucleus. Carbon has 6 protons, so its atomic number is 6; oxygen has 8 protons, so its atomic number is 8. Uranium has 92 protons, so its atomic number is 92! If we count the number of protons plus neutrons, we get an atom's atomic mass. Most elements come in different versions, called "isotopes", with different numbers of neutrons. For example, the most common form of carbon is carbon-12 (12C); that isotope of carbon has 6 protons and 6 neutrons, and thus an atomic mass of twelve. Another isotope of carbon, carbon-14 (12C), has 6 protons and 8 neutrons, hence and atomic mass of fourteen. 12C is radioactive and is used to determine how old things are in a technique called "carbon dating".
Sometimes the electrons get stripped away from an atom. If an atom loses all of its electrons, leaving behind a "naked" atomic nucleus, the nucleus is called an ion. Ions moving at high speeds make up one type of particle radiation. These ions are usually made from relatively small nuclei, like the nucleus of a hydrogen atom (a single proton) or a helium atom (two neutrons and two protons). They can be much larger, though; some cosmic rays are very heavy ions from more massive atoms.