Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Isotope
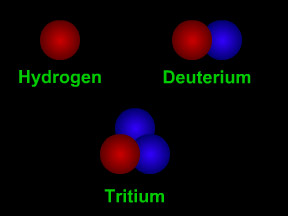
Isotopes are different "versions" of an element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have 6 protons, and all uranium atoms have 92 protons. However, atoms of an element can have different numbers of neutrons. Most carbon atoms have 6 neutrons, but about 1% of the carbon atoms on Earth have 7 neutrons. All carbon atoms have an atomic number (number of protons) of six, but these two different isotopes of carbon have an atomic mass (number of protons + neutrons) of 12 or 13.
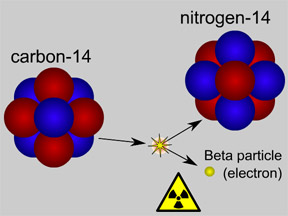
Scientists use special "codes" to write the names of isotopes. One isotope of carbon has 8 neutrons. It has an atomic mass of 14 (6 protons + 8 neutrons). The "code" for this isotope is carbon-14 or 14C. Different isotopes of the same element behave almost exactly the same way in chemical reactions. For example, most oxygen is the isotope oxygen-16. Oxygen-18 is a rare isotope. However, adding two hydrogen atoms to one oxygen atom still makes water (H2O), even if we use 18O instead of 16O. Some isotopes are radioactive, but others are not. Radioactive isotopes can "decay" by giving off radiation.
Where do different isotopes come from? Astronomers think the only elements created in the Big Bang were various isotopes of hydrogen, helium, and probably lithium, beryllium and boron. Supernova explosions created the rest of the elements, including most of their isotopes. Some isotopes form when high-energy cosmic rays crash into atoms in our atmosphere.
Isotopes help scientists learn about the history of some natural events. Carbon dating techniques help us figure out how old some objects are. Oxygen isotopes help us learn about past climates. Some of the water molecules in ice have the rare oxygen-18 isotope in them. The mix of 18O and 16O in ice core samples from Greenland and Antarctica give us clues about Earth's temperature in the past.