Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Atomic Mass
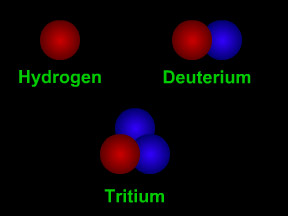
"Atomic mass" is a term physicists use to describe the size (mass) of an atom of a specific type. Since the nucleus of an atom contains nearly all (more than 99%) of an atom's mass, "atomic mass" is more-or-less a description of the mass in the nucleus. The nucleus of an atom contains protons and (in most cases) neutrons. Protons and neutrons have nearly identical masses. "Atomic mass" is essentially a count of the number of neutrons plus the number of protons in the nucleus of a particular type of atom. For example, a simple hydrogen atom with just a single proton has an atomic mass of 1; a "normal" carbon atom with 6 neutrons and 6 protons has an atomic mass of 12.
Sometimes you may see atomic masses expressed as fractions, such as 15.9994 for oxygen. What gives? Surely there isn't a fraction of a proton or neutron hiding inside the oxygen atom's nucleus! The atomic mass for an element is expressed as the weighted average across all isotopes of that element, based on its abundance on Earth. For example, 92% of silicon in Earth's crust is the isotope 28Si, which has 14 protons plus 14 neutrons. However, 5% of silicon atoms have 15 neutrons and another 3% have 16 neutrons, giving them atomic masses of 29 and 30 respectively. The atomic mass of silicon is said to be, therefore, 28.086, when one factors in the masses and relative abundances of different isotopes of silicon.
The behavior of an atom depends more on its atomic number than on its atomic mass. All isotopes of nitrogen behave pretty much the same in chemical reactions, for example. That is because the chemical properties of an element are determined by the number of electrons in its atoms, and the number of electrons equals the number of protons in "normal", neutral atoms. Scientists use the letter "Z" to stand for atomic number and the letter "A" to stand for atomic mass.