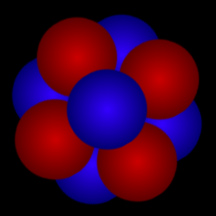
Although the nucleus of an atom is far too small for us to see, here's one way of thinking about an atomic nucleus: as a cluster of tightly packed "balls". The red "balls" represent protons; the blue "balls" represent neutrons. The cloud of electrons that "orbit" an atom's nucleus and define the "size" of an atom is roughly 100,000 times as large as that atom's nucleus!
Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Neutron
A neutron is a sub-atomic (meaning it is smaller than an atom) particle. The nucleus of an atom is made up of neutrons and protons. Neutrons and protons are almost exactly the same size (a neutron has about 1/10th of one percent more mass). A neutron does not have an electrical charge, unlike protons (which have a charge of +1) and electrons (which have a charge of -1). Neutrons are much larger than electrons; the mass of a neutron is about 1,839 times that of an electron!
The number of protons in the nucleus of an atom determines what type of element the atom is. The number of protons is called the element's "atomic number". For example, hydrogen has an atomic number of one, since all hydrogen atoms have one proton in their nucleus. Carbon has 6 protons, so its atomic number is 6; oxygen has 8 protons, so its atomic number is 8. Uranium has 92 protons, so its atomic number is 92! If we count the number of protons plus neutrons, we get an atom's atomic mass. Most elements come in different versions, called "isotopes", with different numbers of neutrons. For example, the most common form of carbon is carbon-12 (12C); that isotope of carbon has 6 protons and 6 neutrons, and thus an atomic mass of twelve. Another isotope of carbon, carbon-14 (14C), has 6 protons and 8 neutrons, hence and atomic mass of fourteen. 14C is radioactive and is used to determine how old things are in a technique called "carbon dating".
Neutrons can exist outside of an atoms nucleus. There is a type of particle radiation called neutron radiation.
Neutrons are made up of even smaller particles called quarks. A neutron is made up of two down quarks and one up quark. Particles, like neutrons, made of three quarks are called baryons.
You might also be interested in:

An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more
The atomic number of an atom tells us how many protons are in the nucleus of that atom. Why is that important? The chemical properties of an element are determined by the number of electrons in its atoms,
...more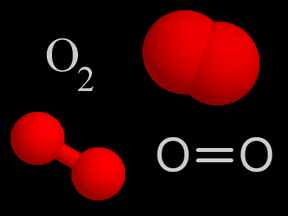
Oxygen is a chemical element with an atomic number of 8 (it has eight protons in its nucleus). Oxygen forms a chemical compound (O2) of two atoms which is a colorless gas at normal temperatures and pressures.
...more
"Atomic mass" is a term physicists use to describe the size (mass) of an atom of a specific type. Since the nucleus of an atom contains nearly all (more than 99%) of an atom's mass, "atomic mass" is more-or-less
...more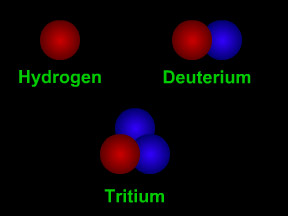
Isotopes are different "versions" of a chemical element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six protons, and
...more
One main type of radiation, particle radiation, is the result of subatomic particles hurtling at tremendous speeds. Protons, cosmic rays, and alpha and beta particles are some of the most common types
...more
Chemistry is the study of matter, energy, and their interactions. Chemists study the composition of substances, their properties, and how they react with each other under varying circumstances. Indeed,
...more