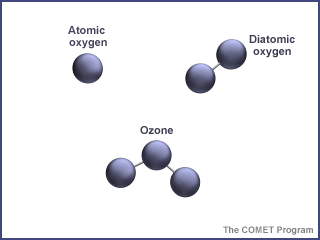
Normal diatomic oxygen molecules (O2) have two oxygen atoms. Ozone (O3) has three oxygen atoms.
Courtesy of COMET program
Ozone
Ozone is a special kind of oxygen molecule. Normal oxygen molecules (O2), the kind we need to breathe, have two oxygen atoms. Ozone molecules (O3) have three oxygen atoms. Ozone forms when a photon of ultraviolet (UV) "light" from the Sun strikes a normal oxygen molecule, breaking it apart. One of the atoms freed by this photodissociation process attaches itself to another oxygen molecule, converting it into ozone.
Ozone occurs naturally in the ozone layer of Earth's atmosphere. It can also be created as a form of air pollution in the lower troposphere. Ozone can irritate the throat and lungs and causes a burning sensation in eyes.
Ozone in the stratosphere protects us from ultraviolet radiation in sunlight. The ozone layer is sort of like sunscreen for planet Earth. It absorbs most of the incoming UV "light" before it reaches the ground. The ozone molecules which absorb UV radiation later re-radiate the energy as heat, warming the stratosphere.
Various chemicals that humans release into the atmosphere can destroy ozone in the stratosphere. This has caused a thinning of the ozone layer in recent years, and even holes in the ozone layer over Earth's poles.
Ozone molecules are much less stable than regular (O2) oxygen molecules. They are very prone to react chemically with other substances. Ozone is also a type of greenhouse gas. Scientists think it is reponsible for about 3-7% of the greenhouse effect on Earth.
You might also be interested in:
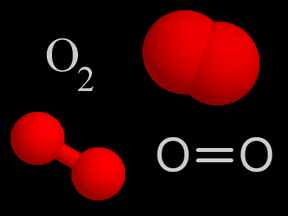
Oxygen (O2) is a kind of gas. A lot of the air you breathe is oxygen. That's a good thing, since we need oxygen to stay alive! About 4/5ths of the air in Earth's atmosphere is nitrogen (N2). Almost all
...more
Respiration is the name of the general process where organisms convert sugars and oxygen into biochemical energy. The process happens in all organisms, including animals, plants, fungi, and bacteria.
...more
Light is very strange. Sometimes it is best to think of light as a series of waves. At other times, it is useful to think of light as a swarm of particles. When we think of light as particles, we call
...more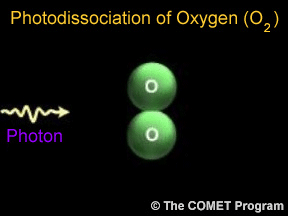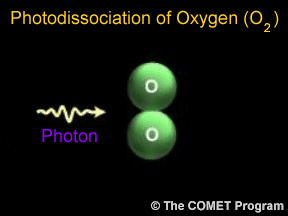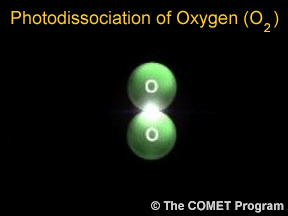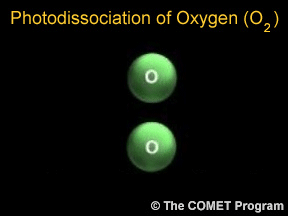
Photons are tiny bits of light and other kinds of electromagnetic radiation. Photons can sometimes break apart molecules. When this happens, it is called photodissociation. When a photon runs into a molecule,
...more
Most things around us are made of groups of atoms bonded together into packages called molecules. The atoms in a molecule are held together because they share or exchange electrons. Molecules are made
...more
Did you know that ozone is found in two different layers of the atmosphere? You may have heard of the ozone hole problem - that is a lack of ozone in the stratosphere (the 2nd layer of the Earth's atomsphere).
...more
About 90% of the ozone in the Earth's atmosphere is found in the region called the stratosphere. This is the atmospheric layer between 16 and 48 kilometers (10 and 30 miles) above the Earth's surface.
...more