This drawing represents a comet bringing atmospheric molecules and possibly primitive life forms to the Earth's surface.
Click on image for full size
JPL/NASA
Acid
Acids are well known as substances capable of dissolving things. If you've ever gotten some battery acid on your clothes and had a hole develop in a couple weeks you'll know what we mean. In this regard, an acid is very much like a
base. Here's a scientific explanation of what an acid is:
An acid is a substance which donates a proton to another species in a reaction. (Donate is really a passive term for what happens. The acid "forces" a proton on a substance which has any capability of accepting it. An acid "donates" a proton the way card players "donate" the Old Maid). Acids are classified as either strong or weak depending upon their relative ability to do this. When it does this, it changes the substance it attacks into a different substance.
There are many acids. Here are a few: Acetic acid (found in viengar), Acetylsalicyclic acid (found in aspirin), Ascorbic acid (found in vitamin C), Citric acid (found in lemon juice), Hydrochloric acid (digestive fluid in the stomach), Sulfuric acid (battery acid). In Earth science however, there only three which really matter because they contribute to weathering of terrestrial rocks. Those are Hydrochloric acid, Sulfuric acid, and Carbonic acid (which plays a role in the Earth's carbon cycle).
You might also be interested in:

We all know that today ocean waters are very salty. Two things help scientists figure out what salt may have been part of the Earth's early oceans. Since there are very little sedimentary rocks of ages
...more
Once the Earth began to cool, water vapor, one of the volatiles, began to condense and form an ocean. According to the Goldilocks theory, Earth is at just the right distance from the sun for the temperature
...more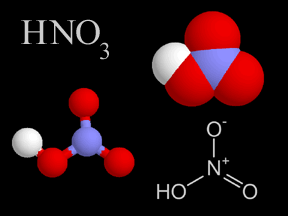
Nitric acid is a very strong acid that can burn your skin. Nitric acid has nitrogen, oxygen, and hydrogen atoms in it. There is a very tiny bit of nitric acid gas in Earth's atmosphere. Nitric acid is
...more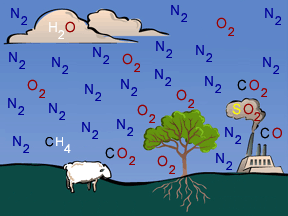
When you think of chemistry, do you think about mixing colored liquids in test tubes and maybe making an explosion... or at least a nice puff of smoke? Did you know that a lot of chemistry happens in Earth's
...more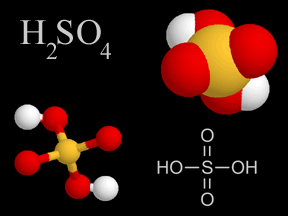
Sulfuric acid is a very common type of acid. Acid rain has sulfuric acid in it. Acid rain harms plants, fish, and other living things. A type of air pollution causes acid rain. When people burn fossil
...more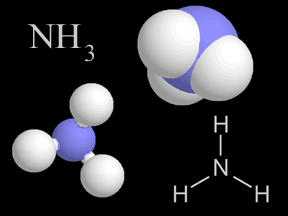
Ammonia is a kind of gas. Ammonia molecules (NH3) have hydrogen and nitrogen atoms in them. The air you breathe has a tiny bit of ammonia in it. When plants and animals die and decay, they give off ammonia.
...more
This page describes extreme environments that are filled with acids, are blasted with radiation, are under high pressure, or are tough places for most living things in various other ways. Extreme environments
...more














