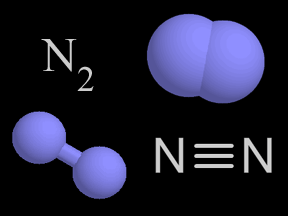A brown haze indicates a combination of dust, nitrogen dioxide, and nitric oxide from car exhaust, power plants and factories.
Click on image for full size
Barry Lefer/MILAGRO
Releasing Nitrogen Pollutants to the Air
While most of the air in our atmosphere is made of nitrogen gas (two atoms of nitrogen bonded together), there are other gases in our atmosphere that contain nitrogen as well. They may make up only a small percentage of the air molecules in our atmosphere, but their numbers are growing and, even in small amounts, they can cause huge changes in our planet.
Nitric oxide (NO) and nitrogen dioxide (NO2)
Nitrogen dioxide and nitric oxide molecules form during combustion in car engines, power plants, and factories. They can contribute to smog when combined with oxygen molecules and the fumes from paint and gasoline (called Volatile Organic Compounds). They can also contribute to acid rain if mixed with water vapor turning into nitric acid. Nitrogen dioxide will break apart in sunlight and the free oxygen atoms latch onto oxygen molecules forming dangerous ground-level ozone.
Nitrous oxide (N2O)
Also known as “laughing gas,” nitrous oxide is a greenhouse gas. The amount of nitrous oxide in the atmosphere has increased since the beginning of the Industrial Revolution.
Nitrous oxide forms during combustion and is also released into the atmosphere from farm animals, sewage, and fertilizers. There are natural ways that nitrous oxide gets into the atmosphere too, including from tiny microbes that chemically alter nitrogen in the soils of tropical forests.
Last modified May 9, 2007 by Lisa Gardiner.
You might also be interested in:
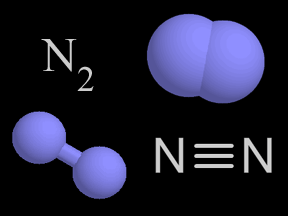
Nitrogen is a chemical element with an atomic number of 7 (it has seven protons in its nucleus). Molecular nitrogen (N2) is a very common chemical compound in which two nitrogen atoms are tightly bound
...more
Most things around us are made of groups of atoms bonded together into packages called molecules. The atoms in a molecule are held together because they share or exchange electrons. Molecules are made
...more
What do smog, acid rain, carbon monoxide, fossil fuel exhausts, and tropospheric ozone have in common? They are all examples of air pollution. Air pollution is not new. As far back as the 13 th century,
...more
Acid rain is a general term used to describe different kinds of acidic air pollution. Although some acidic air pollutants return directly back to Earth, a lot of it returns in rain, snow, sleet, hail,
...more
10% of the ozone in the Earth's atmosphere is found in the troposphere, the first layer of the Earth’s atmosphere. In the troposphere, ozone is not wanted. Ozone is even more scarce in the troposphere
...more
Less than 1% of the gases in Earth's atmosphere are called greenhouse gases. Even though they are not very abundant, these greenhouse gases have a major effect. Carbon dioxide (CO2), water vapor (H2O),
...more
Look up into the sky and you look through millions of air molecules, eighty percent of which are nitrogen molecules, two atoms of nitrogen bonded together. Nitrogen is found all over the planet, not just
...more