A student from the HIGH TIDE project turns on the CTD instrument. High school students use the CTD recorder to measure salinity, temperature and depth of the water in the Lafayette River which is a part of Chesapeake Bay.
Click on image for full size
Image courtesy of the HIGH TIDE project
Salinity - Dissolved Salts, Measuring Salinity
One way to measure how much dissolved salt is in
water is to look at the concentration of salt in the water. Concentration is the amount (by weight) of salt in water and can be expressed in parts per million (ppm). Here are the classes of water:
- Fresh water - less than 1,000 ppm
- Slightly saline water - From 1,000 ppm to 3,000 ppm
- Moderately saline water - From 3,000 ppm to 10,000 ppm
- Highly saline water - From 10,000 ppm to 35,000 ppm
Ocean water has a salinity that is approximately 35,000 ppm. If you take 35,000/1,000,000 then you end up with 3.5%. So, ocean water is about 3.5% salt. Scientists used to report salinity in ppt (parts per thousand), but reporting salinity in ppt is obsolete now as the method of determining ocean salinity in ppt is not used anymore. Instead psu (practical salinity units) are used. Scientists measure salinity by measuring the conductivity of the water as would be measured by a CTD instrument (CTD = conductivity, temperature, depth). Using this method ocean salinity is approximately 35 psu.
Ocean water is about 3.5% salt. That means that if the oceans dried up completely, enough salt would be left behind to build a 180-mile-tall, one- mile-thick wall around the equator. About 90 percent of that salt would be sodium chloride, or ordinary table salt. What other dissolved salts are found in ocean water? The major dissolved salts of the ocean are listed in the following table:
Dissolved salts in
sea water (atoms): |
| 55.3 % Chlorine |
| 30.8 % Sodium |
| 3.7 % Magnesium |
| 2.6 % Sulfur |
| 1.2 % Calcium |
| 1.1 % Potassium |
You might also be interested in:
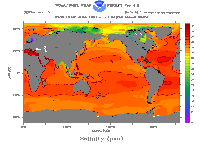
About 70% of the Earth is covered with water, and we find 97% of that water in the oceans. Everyone who has taken in a mouthful of ocean water while swimming knows that the ocean is really salty. All water
...more
Like other types of sedimentary rocks, chemical rocks form at the Earth’s surface, are usually found in horizontal layers, and do not form from molten rock. However, unlike most other sedimentary rocks,
...more
The Phoenix Mars Lander is a space mission sent by NASA to the North Polar Region of Mars. This page describes the instruments aboard the spacecraft and the mission objectives for Phoenix. Click here to
...more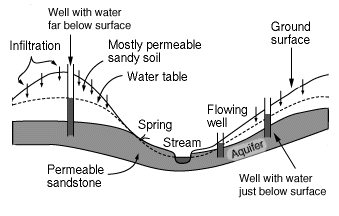
An aquifer is the name for a layer of rock which is capable of holding a large amount of water. Some layers are better at holding water than others, for example a layer of sandstone can hold a good deal
...more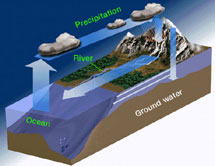
Carbonate is a name for rocks and minerals which contain a certain form of carbon/oxygen compound known as CO32-. (CO32- is also known as the molecule carbonate). Limestone is an example of a calcium carbonate,
...more
One process which transfers water from the ground back to the atmosphere is evaporation. Evaporation is when water passes from a liquid phase to a gas phase. Rates of evaporation of water depend on factors
...more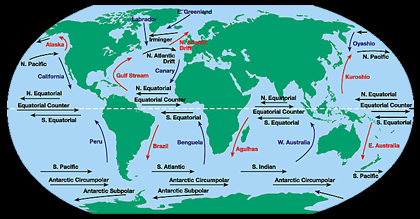
The water at the ocean surface is moved primarily by winds. Large scale winds move in specific directions because they are affected by Earth’s spin and the Coriolis Effect. Because Earth spins constantly,
...more















