This is a picture of the meteorite ALH84001.
Click on image for full size
NASA
The possible discovery of Life on Mars
In July, 1996, it was announced that Dr. David McKay, along with a team of
scientists at Johnson Space Center (a division of NASA), had discovered
possible fossils of
bacteria in an ancient
rock from Mars. The rock is actually a meteorite named ALH84001, which
was found in the Allen Hills in Antarctica in 1984 after having laid around for 12,000 years. While the announcement garnered much excitement at first, upon further study the claims of Martian fossils turned out to be false. NASA said that after two years of study that "a number of lines of evidence have gone away".
The presence of polycyclic-aromatic-hydrocarbons (PAH's), for instance, were originally seen as a possible byproduct of life, but are now viewed as most probably chemicals found on early Mars. Oddly shaped molecules in the meteorite dubbed "worms" resemble many structures created by completely inorganic processes.
Spheres of carbonate were observed in the meteorite which the
scientists in 1996 claimed were the fossilized remains of bacteria. However, they are roughly 1000 times smaller than the smallest bacteria on Earth. Organic compounds were found with the carbonate spheres, but it turned out that the organic compounds became a part of the meteorite after it landed on Earth (scientists believe that there were several episodes of contamination when water seeped into the rock). The carbonate spheres do not contain any carbon 14 (an isotope found on Earth), suggesting that they did not originate on Earth. The organic compounds, on the other hand, do contain carbon 14 and entered the meteorite during an episode of contamination.
The environment of Mars in the
past was very different than it is today. Conditions then may have been favorable for the existence of life. Even though the Mars meteorite did not turn out to be proof of life on Mars, it certainly does not rule out the possibility that life may at one time have existed on Mars.
You might also be interested in:
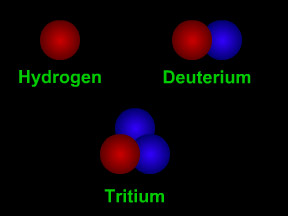
Isotopes are different "versions" of a chemical element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six protons, and
...more
Jupiter's atmospheric environment is one of strong gravity, high pressure, strong winds, from 225 miles per hour to 1000 miles per hour, and cold temperatures of -270 degrees to +32 degrees (freezing temperature).
...more
In July, 1996, it was announced that Dr. David McKay, along with a team of scientists at Johnson Space Center (a division of NASA), had discovered possible fossils of bacteria in an ancient rock from Mars.
...more
Saturn's atmospheric environment is one of strong gravity, high pressure, strong winds, from 225 miles per hour to 1000 miles per hour, and cold temperatures of -270 degrees to +80 degrees. With winds
...more
Titan's atmosphere is a lot like the Earth's, except that it is very cold, from -330 degrees to -290 degrees! Like the Earth, there is a lot of Nitrogen and other complex molecules. There also may be an
...more
Autotrophs are organisms that produce organic compounds from an inorganic source of carbon (carbon dioxide) given a source of energy. If the source of energy is the reactions of inorganic chemical compounds,
...more
In the warm primordial ocean, aggregates of amino acids, proteins, and other hydrocarbons coalesced into a form called *coacervates*. Organic polymers such as amino acids will spontaneously form coacervates
...more














