Click on image for full size
Courtesy of UCAR
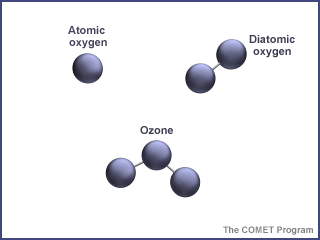
Ozone in the Troposphere
Did you know that ozone is found in two different layers of the atmosphere? You may have heard of the ozone hole problem - that is a lack of ozone in the stratosphere (the 2nd layer of the Earth's atomsphere). But ozone is also found in the troposphere, the first layer of the Earth’s atmosphere. In the troposphere, ozone is NOT wanted! It can actually do a lot of damage.Ozone is released naturally in the troposphere by plants and soil. These are such small amounts that they are not harmful to the health of humans, animals or the environment.
Ozone that increases because of certain human activities does become a problem at ground level and this is what we think of as 'bad' ozone. With increasing populations, more automobiles, and more industry (power plants and refineries in particular), there's more ozone in the lower atmosphere. Since 1900, the amount of ozone near the Earth's surface has more than doubled. In urban areas in the Northern Hemisphere, high ozone levels usually occur during the warm, sunny, summer months (from May through September). Typically, ozone levels reach their peak in mid to late afternoon, after the Sun has had time to react fully with the exhaust fumes from the morning rush hours. A hot, sunny, still day is the perfect environment for ozone pollution production. In early evening, the sunlight's intensity decreases and ground level ozone begins to decrease again.
When ozone pollution reaches high levels, pollution alerts are issued telling people with breathing problems to take extra precautions or to remain indoors. That's no fun! Smog can damage lung tissues, impair an athlete's performance, create more frequent attacks for individuals with asthma, cause eye irritation, chest pain, coughing, nausea, headaches and chest congestion. It can even worsen heart disease, bronchitis, and emphysema.
Rubber, fibers, and certain paints may be damaged by exposure to ozone. Some elastic materials can become brittle and crack (take a look at old rubber bands!), while paints and fabric dyes may fade more quickly.
So why can't we take all of this "bad" ozone and blast it up into the stratosphere where ozone is wanted? Unfortunately, the vehicle necessary to transport such enormous amounts of ozone into the stratosphere does not exist, and, if it did, it would require so much fuel that the resulting pollution might undo any positive effect. We can turn to simpler solutions though, decreasing the production of those chemicals that create ozone in the troposphere. That means choosing to take public transportation instead of all driving separate cars, walking to school or work, and maybe even buying a new hybrid car!?