Click on image for full size
Image courtesy of NOAA, modified by Windows to the Universe staff.
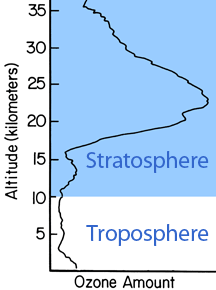
Ozone Layer
The ozone layer is a range of altitudes in Earth's stratosphere which has a higher concentration of ozone molecules. Ozone is an unusual type of oxygen molecule. It is created when high-energy ultraviolet light from the Sun strikes a normal oxygen molecule.
The ozone layer extends from roughly 15 to 35 km (9 to 22 miles) above sea level. The peak of ozone concentration is between 20 and 25 km (12 and 16 miles). Concentrations typically range between 2 and 8 parts per million, though they can rise as high as 15 parts per million. About 90% of the ozone in our atmosphere is contained in the stratosphere. The ozone layer isn't actually a separate layer of Earth's atmosphere; it is a region within the stratosphere.
Ozone in the stratosphere protects us from ultraviolet radiation in sunlight. The ozone layer is sort of like sunscreen for planet Earth. It absorbs most of the incoming UV "light" before it reaches the ground. The ozone layer stops almost all of the incoming UV-C, about 90% of the UV-B, and roughly half of the UV-A radiation. The ozone molecules which absorb UV radiation later re-radiate the energy as heat, warming the stratosphere.
Various chemicals that humans release into the atmosphere can destroy ozone in the stratosphere. That is a problem since it allows more UV radiation to make it to the surface. In the 1980s, scientists noticed that the ozone layer was thinning. They also noticed huge holes in the ozone layer, especially over Antarctica. They convinced people and governments around the world to reduce emissions of ozone-destroying chemicals. They hope the ozone layer will heal itself over time.