Solar Energy in Earth's Atmosphere
Previously, we had assumed a simplified model of Earth; one in which our planet had not atmosphere. This allowed us to examine the basic effects of albedo, latitude, and seasons on our planet's overall average temperature. We'll now add the atmosphere into our figuring; this will complicate matters, but will also make our model more realistic. We will just take a basic look at the atmosphere's influence on incoming solar radiation; we won't discuss winds and circulation patterns at this point.
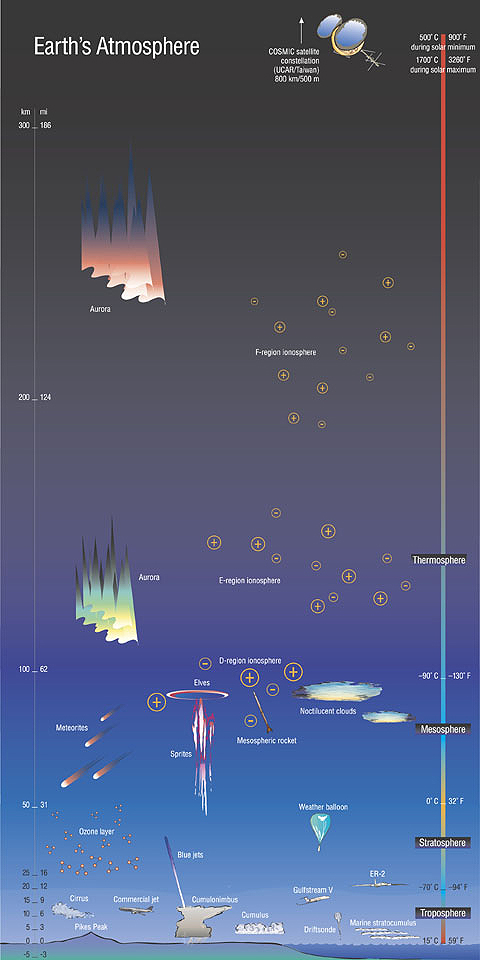
Review: Layers of Earth's AtmosphereYou probably know that scientists think of our atmosphere as having several distinct layers with specific traits. Let's quickly review the structure of the atmosphere, since some aspects of that story are relevant to how and where solar energy gets absorbed. Nearest ground level is the troposphere. It extends upward from the ground to an altitude of about 16 km (in the tropics, or 8 km near the poles). Most weather occurs, and most clouds are to be found, in this layer. Convection currents keep the gases in the troposphere well mixed. The troposphere is warmest near ground level, and cools gradually the higher up in it one goes. Immediately above the troposphere lies the stratosphere. Some high-altitude clouds can be found in this layer. Temperatures actually increase with altitude as one moves upward through the stratosphere. Jet airliners fly in this layer, for it is far less turbulent than the underlying troposphere. The stratosphere extends upward from the top of the troposphere to an altitude of about 50 km. The ozone layer lies within the stratosphere. Moving upward, the next layer is the mesosphere. By this point the atmosphere is very thin. Temperatures once again decline with increasing altitude (as was the case in the troposphere), falling as low as -100° C (-146° F) in the upper mesosphere. This layer is relatively poorly studied, for it is above the reach of most aircraft but below the altitude where satellites orbit. Most meteors burn up in the mesosphere. The top of the mesosphere lies about 80 to 85 km above Earth's surface. Above the mesosphere lies the extremely tenuous thermosphere. This layer is so thin, in fact, that many satellites orbit within it. This region is one in which temperatures once again rise with increasing altitude, reaching as high as 2,500°C (4,500°F) in the daytime! Embedded within the thermosphere are several layers of the ionosphere; regions where ionized gas particles can reflect radio waves, a feature that people used to send messages beyond the line-of-sight range of the horizon before the advent of satellites. The thermosphere extends to somewhere between 500 and 1,000 km above the Earth's surface. Many of the atoms and molecules in the thermosphere (and above) have lost electrons, thus becoming electrically charged ions; so the motions of particles in the upper atmosphere are partially influenced by electrical currents and Earth's magnetic field. Though not universally recognized as a layer of our atmosphere, some scientists consider the exosphere to be the outermost layer of Earth's atmosphere. Starting at the top of the thermosphere, this extremely tenuous layer gradually gives way to the vacuum of interplanetary space. | ||
Solar EM Radiation Penetration into Earth's Atmosphere
Let's now take a look at the electromagnetic radiation, of various wavelengths and energies, from the Sun as it penetrates into Earth's atmosphere. Recall that the Sun emits a broad range of frequencies, from high-energy X-rays and ultraviolet radiation, through visible light, on on down the spectrum to the lower energy infrared and radio waves. Different wavelengths of this solar radiation behave differently as they enter our atmosphere. All of the high-energy X-rays are absorbed by our atmosphere well above our heads, which is very fortunate for us indeed! Likewise, most of the UV radiation (especially the highest energy, shortest wavelength regions of the UV spectrum) is blocked by the thermosphere, mesosphere, and stratosphere. The relatively low energy, long wavelength portion of the UV spectrum that does reach the ground forces us to wear sunglasses and slather ourselves with sunscreen to protect ourselves from sunburn and skin cancer. A relatively narrow "window" of EM wavelengths around visible light reaches the ground. It includes some of the longer wavelength UV frequencies, some of the shorter wavelength IR frequencies, and all of the visble light region of the spectrum. Most of the longer wavelength IR waves, and many of the shorter radio waves, are absorbed by the stratosphere before reaching the ground. There is a sizeable "radio window" of radio wave frequencies that also reach terra firma. The longest wavelength radio waves also fail to penetrate the atmosphere; many are absorbed or reflected by the ionosphere. Recall how the temperature of the various layers of Earth's atmosphere rises and falls as one moves upward from the ground, in a seemingly haphazard fashion. First the temperature drops as we move upward through the troposphere; then it rises as we rise through the stratosphere; then it falls again through the mesosphere, only to rise again in the thermosphere. What's going on here? Near the Earth's surface, the sunlight that does reach the ground warms the Earth, which in turn warms the air immediately above it. So the troposphere is warmest next to the warm ground, and cooler higher up away from the warm ground. However, in the stratosphere, our friend the ozone layer is especially good at absorbing UV radiation; which shields us from most of these high-energy rays, and also heats this layer as the UV photons transfer their energy to the oxygen (ozone is an unusual type of oxygen molecule, O3) molecules. And so it goes, throughout our upper atmosphere. The air varies in its chemical composition at different altitudes; and various chemical species absorb different wavelengths of EM radiation preferentially. Wherever there is the right combination of certain chemicals and an abundance of radiation of a type that those chemicals are good at absorbing, the atmosphere absorbes a lot of energy and its temperature rises. Remember how certain images of the Sun as certain specific wavelengths, especially in various narrow bands of the UV spectrum, provided us with "views" of very specific elements (such as helium or iron) in the Sun? Just as certain elements emit specific wavelengths of EM radiation, certain elements and compounds preferentially absorb certain specific wavelengths. In this sense, Earth's atmosphere is sort of the "flip side" of the Sun's atmosphere.
|
||
The Greenhouse EffectMost of the solar radiation that reaches the Earth or its lower atmosphere, where albedo from clouds and features on the ground come into play, is thus in the form of visible light. So what is the fate of that sunlight? Recall that, averaged over the surface area of Earth's globe, about 342 W/m2 of energy reaches the top of Earth's atmosphere. Recall also that the peak of the Sun's EM emissions are in the visible light region of the spectrum. So, although the atmosphere blocks out much of the range Sun's range of EM emissions at various altitudes, the bulk of the EM radiation from the Sun in the form of visible light does reach at least as far as the troposphere. Earth's overall average albedo, including clouds, is around 0.3 (or 30%, if you prefer). So about 30% of the incoming sunlight is reflected back into space by clouds or light areas on Earth's surface, or scattered back out into space by gas molecules in the atmosphere (that scattering is what makes the sky blue, not black!). Of the 70% that makes it through, about 2/3rds makes it to the ground, while about 1/3rd is absorbed (not reflected) by clouds. As you'd expect, when the ground (or a cloud) absorbs sunlight, it heats up. Recall that anything that is warmer than absolute zero emits infrared radiation. So both the ground and the clouds emit in IR wavelengths. At this point a critical player in the climate drama enters the scene - the Greenhouse Effect! As was demonstrated in the "Infrared - More Than Your Eyes Can See" video earlier, some substances are transparent to certain wavelengths of EM radiation, but largely opaque to others. Such is the case with the panes of glass in a greenhouse; sunlight readily passes in, providing plants with the energy they need for photosynthesis and warming the inside of the greenhouse, but the IR radiation that the warmed interior emits does not readily pass back out through the glass, for the glass is largely opaque at infrared frequencies. Thus, the greenhouse traps in the IR radiation and stays quite warm, even on a winter day. Earth's atmosphere is much like the glass in the greenhouse. Our atmosphere is opaque across much of the IR spectrum; take a look again at the image above that shows how much of the incoming IR radiation from the Sun fails to make it to the ground. On the way in, most of the EM radiation is in the form of visible light, which easily passes through clear air. On the way, back up, however, much of the radiation is in the form of infrared, which is absorbed and thus stopped before it reaches space. Earth's atmosphere, like the panes of glass in the greenhouse, traps much of the infrared radiation, and the heat that it carries, warming our planet. Several different gases play a role in this greenhouse effect; water vapor, carbon dioxide, and methane are amongst the most prominent. As infrared radiation from the ground and low-lying clouds works its way up through the atmosphere, much of it is absorbed; by gases in the air and by clouds (which have lots of IR-opaque water). The atmosphere and clouds warm, once again emitting infrared radiation. Some of that radiation goes upward, escaping into space; but some goes downward, further warming the ground and the lower atmosphere. This cycling of the IR radiation's energy through the lower atmosphere warms our planet to a much more comfortable temperature than the frigid range that we calculated for an airless world. Recall that our airless Earth was expected to have an average temperature around -19° C (-3° F). In actuality, Earth's overall average temperature is somewhere around +15° C (+60° F). The greenhouse effect (in moderation!), is what makes our planet habitable. Without it, we'd be living on an iceball (if we were here at all!). |
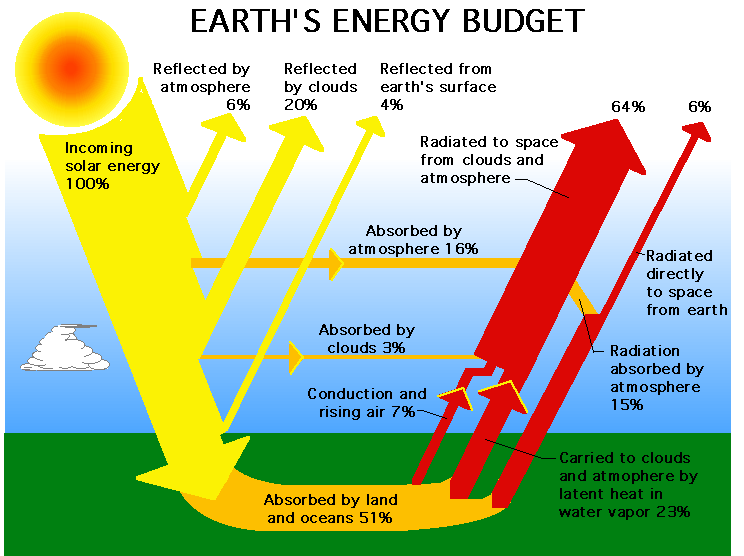
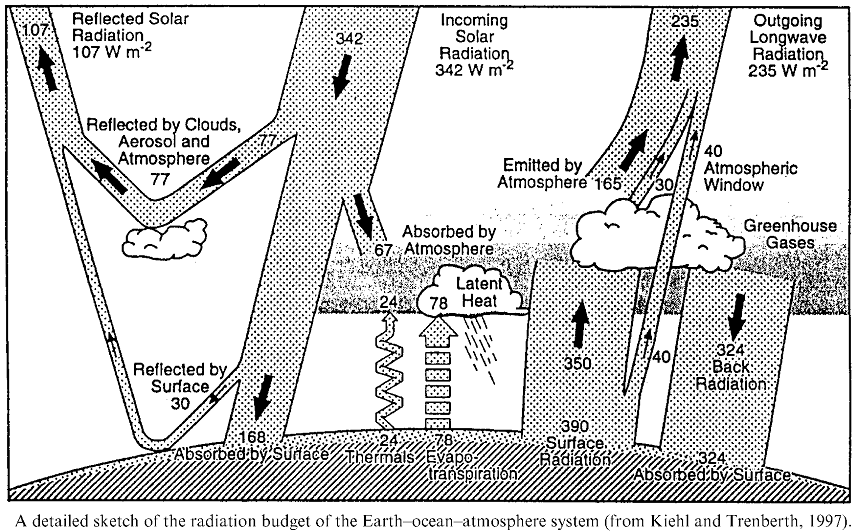
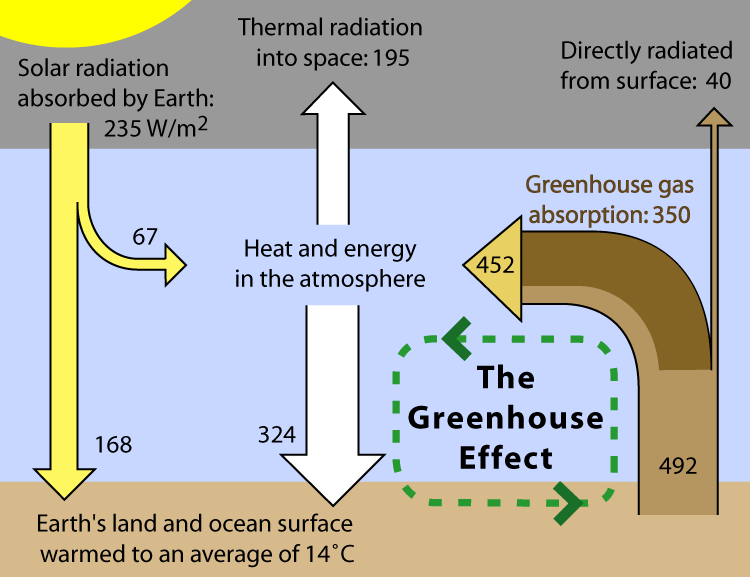
Here (below) are three different diagrams illustrating the flow of visible light into, and infrared light back out of, the lower regions of Earth's atmosphere. I can't say that I find any one of them ideal; each seems to have some confusing aspects, and I'm not sure where the one gets its value of 235 W/m2 of incoming solar radiation from. I think each diagram has its strengths in helping to tell parts of the story; and I'll let you choose which (if any) you think might be most helpful when presenting these ideas to your students.
Diagram of Earth's energy budget. |
Diagram of Earth's radiation budget. |
Diagram illustrating the greenhouse effect . |