ExploraTour - Looking at the World in a Different Light
Click on image for full size
Windows Original.
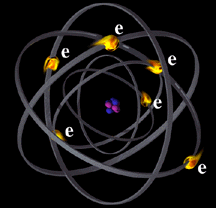
Protons are positively charged, electrons are negatively charged and neutrons have no charge. As you look down at the nucleus, you see that there are the same number of protons below you as there are electrons whizzing around above you.
Since opposite charges attract each other, the electrons are held in orbit about the nucleus by the force of their electromagnetic attraction to the positively charged protons. But as seen from outside the atom, the negative charges of the electrons exactly balance the positive charges of the protons making the atom electrically neutral.
Electrons can be knocked out of their orbits by collisions or interactions with radiant energy . The atom, now called an ion, is left with an excess of positive charge.
The secret behind radiant energy lies in the movement of charged particles within this subatomic world.