ExploraTour - Looking at the World in a Different Light
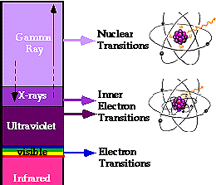
Light we can see (visible) is produced when electrons change orbits within atoms or molecules. Outside the range of human vision and to the far side of violet lies radiation with higher energies and shorter wavelengths than visible light. This "ultraviolet" radiation (called "black light" because it can't be seen) results from energy changes in the orbiting electrons in atoms that make up very hot substances.
X-rays are produced when tightly-bound electrons, close to the nucleus, change orbits due to violent collisions or other energetic processes, but they can also be generated when electrons slow down very fast. Gamma rays result from high energy processes that affect the atomic nucleus itself such as nuclear fusion.
Moving beyond red light brings us to lower energies and longer wavelengths than visible light. "Infrared" radiation (called heat radiation - remember the bed?) results from changes in the loosely-bound electrons far from the atomic nucleus and from changes in the motions of molecules. The atoms and molecules of objects at normal temperatures on Earth emit radiation in this range.
Atoms and molecules that make up matter are constantly in motion, changing their energy states and emitting radiation. The amount and frequency of radiation decreases until the temperature of the object reaches "absolute zero", when theory predicts all of these movements stop.
Very cool objects emit part of their radiation at microwave or radio frequencies. As the temperature increases, the amount and frequency of radiation increases. Very hot objects emit part of their radiation in the X-ray, UV or visible range.