
Name________________________
Background
The ocean-atmosphere interface is one place where exchanges take place all the time. What if we change the balance of what is exchanged? For example, what if we increase the amount of carbon dioxide in the atmosphere – will it impact the amount of carbon dioxide that makes its way into our oceans? What will happen to the chemical balance of ocean water and the marine life living there? In this investigation, you will explore this exchange of gases and identify the changes in the chemical balance of seawater.
Lab Question
How does the presence of carbon dioxide change the pH of water? How has the increase in atmospheric carbon dioxide changed the chemistry of our oceans?
Materials per lab team
Procedure
Part 1: Detecting CO2 Gas

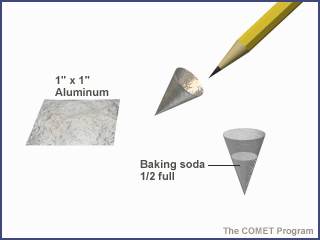
Figure 1

Figure 2
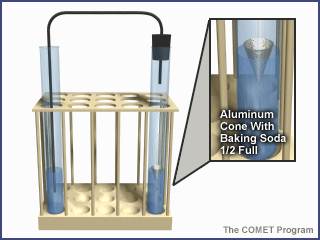
Figure 3
Part 2: Are animals a source of CO2?
Part 3: Are plants a source of CO2?
Part 4: Do Plants take up CO2?
Part 5: Are Fossil Fuels a Source of CO2?
Data Table 1:
|
|
BTB Color Change |
Acid or Base |
What Happened?
|
|
Part 1 |
From:
To: |
|
|
|
Part 2 |
From:
To: |
|
|
|
Part 3 |
From:
To: |
|
|
|
Part 4 |
From:
To: |
|
|
|
Part 5 |
From:
To: |
|
|
Part 6: pH and Marine Ecosystems
Date Table 2:
|
|
Color |
pH |
What Happened? |
|
pH indicator solution + water |
|
|
***Initial color*** |
|
pH indicator solution + water + breath |
|
|
|
|
pH indicator solution + water + breath + shells/coral |
|
|
|
Analysis
1. After finishing all the parts of this activity, compare the colors in all the tubes. Are they different? Describe the phenomena that took place in each test tube and the beaker/cup.
A.
B.
C.
D.
E.
Beaker/cup:
2. What happens when carbon dioxide enters ocean water? Refer to your lab results.
What happened to the pH of the solution after you added the shells?
What happened to the shells or coral after they remained in the solution for a little while?
Based on what happened in this investigation, can the same effects happen in our oceans to marine life with shells or calcium carbonate exoskeletons? Explain your answer.
Conclusion
1. Use this image of the carbon cycle and the terms sources and sinks to write a paragraph about the processes in the carbon cycle.
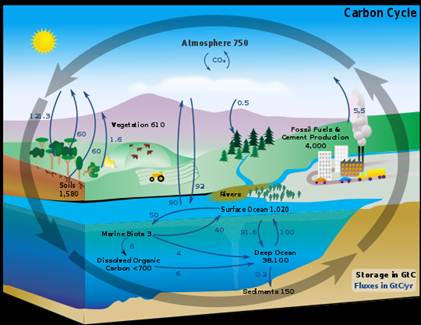
Figure 4: This
carbon cycle diagram shows the storage and annual exchange of carbon between
the atmosphere, hydrosphere and geosphere in gigatons - or billions of tons -
of Carbon (GtC).
(Source: Wikipedia
Commons)
2. What is meant by the term ocean acidification?
3. When ocean acidification occurs, calcium carbonate molecules dissociate causing a loss in shells, exoskeletons, or coral reefs. Research a marine organism (other than coral) which relies on calcium carbonate for its exoskeleton. Use the results of this lab to predict what would happen if our oceans became more acidic.
Application
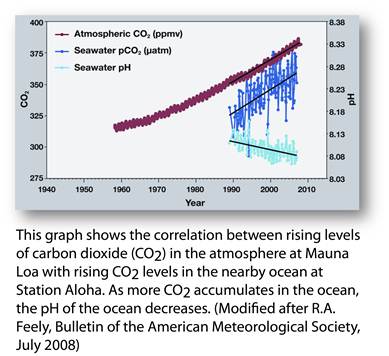
Figure 5
(Source: NOAA PMEL
Carbon Program
http://www.pmel.noaa.gov/co2/file/Hawaii+Carbon+Dioxide+Time-Series)
1. Study the graph above. What is the overall relationship between the atmospheric carbon dioxide and the amount of carbon dioxide in the oceans?
2. What happens to the pH of seawater as more and more carbon dioxide dissolves in the seawater?
3. What implications does this have for marine life around the globe?
4. How will this affect populations relying on the marine life for food and their economy?
5. What can be done to reverse the trend in this graph? Explain why you think this could work? What would the challenges be to making it work?