Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Atomic Number
The atomic number of an atom tells us how many protons are in the nucleus of that atom. Why is that important? The chemical properties of an element are determined by the number of electrons in its atoms, and the number of electrons equals the number of protons in "normal", neutral atoms. Each element has a different number of protons in the nuclei of atoms of that element; so each element has a different atomic number.
Hydrogen atoms have 1 proton, and thus an atomic number of 1. Carbon has 6 protons and an atomic number of 6; oxygen has 8 protons and thus and atomic number of 8. The atomic number of uranium is 92!
Scientists also use the concept of "atomic mass". Since the nucleus of an atom contains nearly all (more than 99%) of an atom's mass, "atomic mass" is more-or-less a description of the mass in the nucleus. The atomic mass of an atom is essentially a count of the number of neutrons plus the number of protons. Common carbon has 6 protons and 6 neutrons in each carbon atom, so its atomic mass is 12 ( = 6 + 6). Sometimes scientists use the letter "Z" to stand for atomic number and the letter "A" to stand for atomic mass.
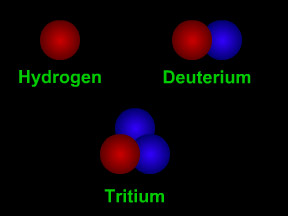
Most elements have different "versions" with varying numbers of neutrons. The different versions are called isotopes. Carbon, for example, has isotopes with 7 neutrons and with 8, along with the standard 6-neutron variety. Scientists specify which isotope they are talking about by including the atomic mass in the name. Normal carbon is thus carbon-12, while the less common varieties are written as carbon-13 and carbon-14. Remember, however, that the different isotopes of carbon behave almost identically in most chemical reactions, for they share the same atomic number.