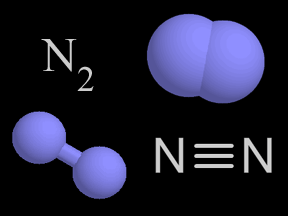Click on image for full size
Image courtesy UCAR, modified by Windows to the Universe staff (Randy Russell).
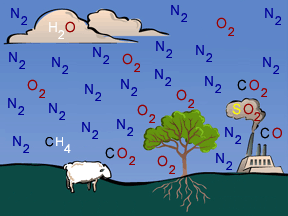
Atmospheric Chemistry of Earth's Troposphere
Chemistry plays an influential role in the behavior of the lowest layer of Earth's atmosphere, the troposphere. The various gases in the troposphere are constantly mixing with and reacting with each other. Gases released by the oceans, emitted by living creatures, and poured into the atmosphere by human activities continually alter the balance of atmospheric chemistry.
Earth's atmosphere consists of about 78% nitrogen, 20% oxygen, and a mixture of small amounts of numerous other ingredients. Some of the minor constituents do, however, have big impacts. For example, greenhouse gases such as carbon dioxide and methane exert a large influence on the temperature of our planet.
The activities of humans play an increasingly important role in tropospheric chemistry. Fossil fuel burning generates sulfur oxides, which create sulfuric acid - a component of acid rain. Exhaust gases from cars and trucks produce nitrogen oxides, which contribute to the formation of smog and of nitric acid - another component of acid rain.
Nature also does its share to alter the chemistry of the troposphere. Volcanic eruptions, wildfires, lightning, and UV radiation from the Sun all add materials to the troposphere or chemically alter those already there. The oceans and the biosphere exchange vast quantities of gases with the atmosphere's lowest layer. The Carbon and Nitrogen Cycles play key roles in these processes.
The table below describes some of the chemical compounds that play important roles in the chemistry of Earth's troposphere. It also lists some of the processes that transform those chemicals.
| Chemical Compound | Formula | Role in Tropospheric Chemistry |
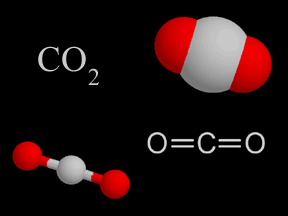
| Carbon dioxide | CO2 |
Carbon dioxide in the troposphere is produced by photosynthesis by plants and microbes, by decomposition of organic matter, and by fossil fuel combustion. CO2 is an important greenhouse gas. |
| Carbon monoxide | CO |
Carbon monoxide comes from wildfires, volcanoes, and incomplete combustion in the exhaust gases from cars and trucks. It is poisonous and can help raise the levels of greenhouse gases via certain chemical reactions. It reacts with oxygen to transform into carbon dioxide. |
| Hydrocarbons | CxOy |
Hydrocarbons are released by the combustion of fossil fuels. They are one of the constituents of smog. Hydrocarbons are combinations of hydrogen and carbon atoms. |
| Hydrogen Peroxide | H2O2 |
Hydrogen peroxide, often present in small quantities in water droplets in the atmosphere, helps create the sulfuric acid in acid rain via reactions with sulfur dioxide. |
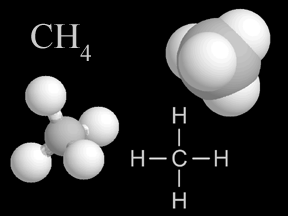
| Methane | CH4 |
Methane is a powerful greenhouse gas. Although its concentration in the atmosphere is small, it has a substantial impact on Earth's energy balance. |
| Nitrogen | N2 |
78% of the gas molecules in the troposphere are nitrogen. Nitrogen atoms move through the environment via the nitrogen cycle. Hot combustion, such as in auto exhausts, incorporates nitrogen into nitrogen oxides. |
| Nitrogen Oxides | NO & NO2 |
Nitrogen oxides form during high-temperature combustion in air, such as in automobile exhausts. They help to form smog and mix with water to create nitric acid, a component of acid rain. |
| Nitric Acid | HNO3 |
Nitrogen oxides from pollutants such as car exhaust mix with water to form nitric acid. It is a component of acid rain. |
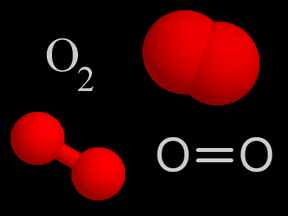
| Oxygen & Ozone | O2 & O3 |
About 20% of the gas molecules in the troposphere are oxygen. Although ozone in the stratosphere is good and protects us from UV radiation, ozone in the troposphere is bad and is a health hazard that contributes to the formation of smog. |
| PAN (Peroxyacytyl nitrate) | C2H3O5N |
PAN is a noxious and irritating component of smog. It forms in a reaction involving nitrogen dioxide, oxygen, and substances derived from Volatile Organic Compounds (VOCs). |
| Photochemical smog | - |
Smog was originally a term for a mixture of smoke and fog. Photochemical smog is a toxic "soup" of atmospheric pollutants often found in urban areas. It consists of nitrogen oxides, ozone, VOCs, and PAN. |
| Photodissociation | - |
When molecular bonds break due to energy from a collision with a solar photon. |
| Sulfur Oxides | SO2 & SO3 |
Sulfur dioxide and sulfur trioxide are produced by coal and oil burning, volcanoes, and other human and natural sources. Sulfur dioxide combines with water droplets in the air to form sulfuric acid, a component of acid rain. |
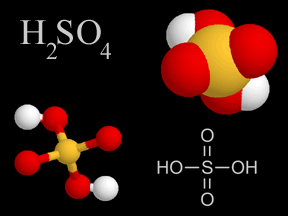
| Sulfuric Acid | H2SO4 |
Sulfur dioxide gas from coal and oil burning, volcanoes, and other human and natural sources, combines with water droplets in the air (and the traces of hydrogen peroxide such droplets often contain) to form sulfuric acid - which is a component of acid rain. |