Aerosols, Cloud Nucleation and Global Dimming
 Global warming induced by greenhouse gases gets most of the press coverage, but it is not the only climate change issue our planet is dealing with. Emissions of tiny particles, called aerosols, into our atmosphere are disrupting our climate as well. Aerosols come from both natural sources (such as dust storms, volcanic eruptions, and wildfires) and anthropogenic sources (primarily coal-fired power plants and internal combustion engines in cars and trucks). Aerosols alter Earth's energy budget (some scatter or reflect light, while others are strong absorbers of solar energy) and cause changes to the water cycle (aerosols play a critical role in cloud and raindrop formation). The overall effects of aerosols are complex, for there are many different types that influence several aspects of climate in a variety of ways.
Global warming induced by greenhouse gases gets most of the press coverage, but it is not the only climate change issue our planet is dealing with. Emissions of tiny particles, called aerosols, into our atmosphere are disrupting our climate as well. Aerosols come from both natural sources (such as dust storms, volcanic eruptions, and wildfires) and anthropogenic sources (primarily coal-fired power plants and internal combustion engines in cars and trucks). Aerosols alter Earth's energy budget (some scatter or reflect light, while others are strong absorbers of solar energy) and cause changes to the water cycle (aerosols play a critical role in cloud and raindrop formation). The overall effects of aerosols are complex, for there are many different types that influence several aspects of climate in a variety of ways.
Let's take a look at these aerosols, with an eye towards their impact on Earth's climate. Here are the key points we'll delve into:
- What are aerosols? What are the different types of aerosols? Where do they come from? (both natural and anthropogenic sources)
- What roles do aerosols play in Earth's atmosphere? They act as cloud condensation nuclei, they alter albedo (both directly and indirectly via clouds) and hence Earth's radiation budget, and they serve as catalysts of or sites for atmospheric chemistry reactions.
- What is "global dimming" and what role do aerosols play in this phenomenon?
Aerosols, the basics: Sources and Types
Aerosols are small particles that drift aloft in our atmosphere. Aerosols are not gases. Aerosols are usually solids, though some are tiny droplets of liquid. Aerosol particles are sufficiently small and light that they do not quickly fall out of the air under the influence of gravity; some aerosols remain aloft for a few hours, while others can stay airborne for years.
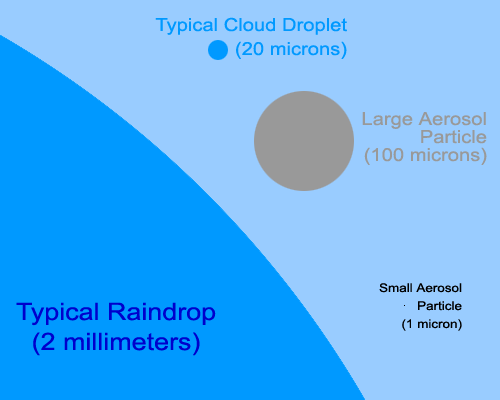
How small are aerosols? They range in size from about 10 nanometers to 100 microns in diameter. The smallest particles are only a few molecules across, while the largest are roughly the same width as a strand of human hair. A typical raindrop is about 2 millimeters (2,000 microns) in diameter, while a typical cloud droplet is about 20 microns across.
This cartoon shows the relative sizes of a typical raindrop, a droplet of water in a cloud, an extremely large aerosol particle, and a smaller aerosol particle of a more typical size. Even smaller aerosol particles exist; some as small as 10 nanometers (0.01 microns) across. Those particles would be invisible at even this magnified size scale. |
Scientists use a few different schemes to classify aerosols; by their source or method of formation, by the way they interact with sunlight, and by their chemical properties. Let's first look at the major sources of aerosols.
Aerosols are generated both naturally and as a result of human activities. Dust, mostly in the form of fine mineral particles, is the most prevalent natural source of aerosols. Large wind storms over deserts and other arid regions can lift dust particles high into the atmsophere, allowing them to drift downwind hundreds of kilometers from their source. Sea spray accounts for the second most common natural source of aerosols in the form of various types of salts. These aerosols mostly fall back into the ocean near the place where they were emitted. Large volcanic eruptions spew vast clouds of fine ash particles into the air, sometimes reaching the stratosphere. Ash from very large eruptions can stay aloft for months to a few years, and can spread around the globe. Forest and grassland fires create soot particles as part of the smoke they emit. Marine phytoplankton are also responsible, indirectly, for the production of large amounts of aerosols. We'll explain this further when we describe primary emissions and secondary emissions of aerosols. Likewise, land-based vegetation emits gases, such as volatile organic compounds (VOCs), that contribute to secondary emissions of aerosols.
Anthropogenic (human produced) aerosols make up about 10 percent of the amount of aerosols in our atmosphere. Tiny particles of black carbon, or soot, are a major component of smoke produced by many kinds of burning. Coal-burning power plants generate lots of black carbon and often loft it high into the atmosphere as emissions from tall smokestacks. Internal combustion engines in cars, trucks, and construction vehicles also emit plenty of black carbon. Diesel engines are especially prolific producers of this type of aerosol. Humans activities also increase the amount of mineral dust aerosol generation. Desertification, the removal of plants that help prevent wind erosion of soil, and large construction sites are the major anthropogenic sources of dust aerosols. Finally, pollutant gases such as sulfur oxides and nitrogen oxides, which are emitted by fossil fuel combustion and a variety of industrial processes, can generate secondary emissions of aerosols as a result of chemical reactions in the atmosphere.
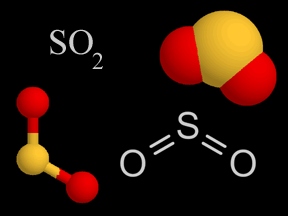
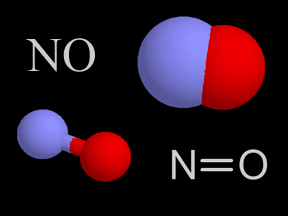
What is the concept of primary emissions versus secondary emissions of aerosols all about? As you might guess, primary emissions are aerosol sources that directly project aerosol particles into the air. Dust, volcanic ash, and black carbon from various types of burning are all examples of primary emissions. Secondary emissions refers to substances that are not aerosols when they are originally emitted, but later undergo some chemical reaction in the atmosphere that transforms them into aerosols. For example, sulfur dioxide (SO2) gas is emitted by volcanoes, forest fires, coal-burning power plants, refuse incineration, and other sources. In the atmosphere, chemical reactions and combination with water can transform sulfur dioxide into sulfuric acid droplets, a liquid aerosol that helps form acid rain. Sulfuric acid can also combine with gaseous ammonia (NH3) to form a solid ammonium salt, ammonium sulfate ( [NH4]2[SO4] ). Thus emissions of gaseous sulfur dioxide can eventually produce multiple aerosols, in both liquid and solid states. Sulfur oxide and nitrogen oxide gases, generated by natural and anthropogenic processes, are the most prevalent sources of secondary emissions of aerosols. Exposure to solar radiation, often in the form of ultraviolet photons and sometimes at high altitudes, is frequently integral to the transformation of these gases into aerosols.
Sulfur dioxide (top) and nitric oxide (center) are two gases that are often transformed into aerosols (secondary emissions). Chemical reactions in the atmosphere convert these (and some other) gases into liquid or solid aerosols. In many cases, solar electromagnetic radiation (often high-energy ultraviolet photons) supplies the energy needed to inititate these reactions. The animation (bottom) illustrates the photodissociation of a nitrogen molecule. Credits: The depictions of the two molecules (top and center) are original artwork by Windows to the Universe staff (Randy Russell). The photodissociation animation (bottom) was provided by The COMET Program. |
What roles do aerosols play in Earth's atmosphere?
The presence of aerosols in Earth's atmosphere influences climate in three key ways. Aerosols alter albedo, changing the amount of solar energy that reaches the planet's surface and the amount that is absorbed at various levels within the atmosphere. Aerosols also play a large role in the formation of clouds of various types and at various altitudes. Finally, aerosols help to enhance or suppress various types of chemical reactions in the atmosphere, producing important but difficult to study effects on the climate forcings of many compounds present in the atmosphere.
Different aerosols interact with sunlight (and other electromagnetic radiation) in various ways. Sea salt does not absorb sunlight, but mineral dust does. Black carbon, as you might guess, is a strong light absorber. All aerosols, including sulfate and nitrate aerosols, scatter light to some extent. Aerosols of different types can, therefore, influence climate in one or more ways. Many aerosols scatter incoming sunlight back out into space, effectively increasing Earth's albedo and exerting a cooling effect on the planet. However, aerosols that also absorb sunlight (especially black carbon) effectively increase albedo warming the atmosphere in their vicinity when the reradiate the absorbed energy in the infrared portion of the spectrum. Note that such absorption and heating may occur near Earth's surface or high above it in the stratosphere, and that the location of that heating can make a big difference in terms of the overall effect on climate.
Aerosols play a critical role in the formation of clouds. Clouds form as parcels of air cool and the water vapor in them condenses, forming small liquid droplets of water. However, under normal circumstances, these droplets form only where there is some "disturbance" in the otherwise "pure" air. In general, aerosol particles provide this "disturbance". The particles around which cloud droplets coalesce are called cloud condensation nuclei (CCN) or sometimes "cloud seeds". Amazingly, in the absence of CCN, air containing water vapor needs to be "supersaturated" to a humidity of about 400% before droplets spontaneously form! So, in almost all circumstances, aerosols play a vital role in the formation of clouds.
|
As we have seen, not all aerosols are the same. As you might expect, different aerosols play different roles in cloud formation. Aerosols come in different sizes and are lofted to different heights in the atmosphere. Different types of clouds exist at different heights. Some clouds have smaller droplets than others; clouds with smaller droplets have a higher albedo, while clouds with larger droplets are more prone to producing precipitation. Temperature and humidity play key roles in determining the types of clouds that form in a given locale, but so too do aerosols. The abundance, sizes, and types of aerosols present all factor into cloud formation. Any disturbance to the normal mix of aerosols, whether from natural events like volcanic eruptions or wildfires, or from anthropogenic ones like emissions from fossil fuel burning, tends to alter the types and numbers of clouds that appear in that region or downwind of it. Changes to clouds alter solar energy input via an altered albedo, alter precipitation patterns, and alter the strength of the greenhouse effect. These changes affect large areas, but are not uniform on a global scale. One area might see more clouds, another fewer, and another a changed abundance of high altitude clouds. Such changes impact climate in important, but complex, ways.
Finally, the chemical properties of aerosols can play a large role in their abilities to influence climate. Some are relatively inert, others are highly reactive, and some react strongly only with certain substances. Chemical reactions involving aerosols can generate new substances that influence climate, or they can diminish the amounts of certain other chemicals in the atmosphere, again altering the existing balance. Reactions can cause aerosols to grow in size, altering their ability to absorb or scatter light or other electromagnetic radiation. Aerosols can also serve as platforms upon which other substances accumulate, increasing the rates of certain chemical reactions that would otherwise be too infrequent to matter because of low rates of molecular collisions. The details of atmospheric chemistry involving aerosols are complex, and scientists are actively researching this field today.
What is "global dimming" and how are aerosols involved?
Since the 1950s scientists have been making systematic measurements of the amount of sunlight reaching Earth's surface. They discovered that the average worldwide irradiance from the Sun decreased by about 4% over the three decades from 1960 to 1990. This phenomenon has been dubbed "global dimming".
Scientists believe that aerosols emitted as a result of human activities are the most likely cause of global dimming. Recall that some aerosols scatter incoming sunlight back into space, while others (especially black carbon) absorb sunlight. Also, since aerosols serve as cloud condensation nuclei, an increase in aerosols could generate more clouds. Clouds, of course, have a very high albedo, and thus tend to reflect even more incoming sunlight back into space. In each case, some sunlight would be prevented from reaching Earth's surface; the result would be an apparent dimming of the Sun as viewed from the planet's surface.
|
A reduction of sunlight at Earth's surface tends to reduce the surface temperature. So global dimming has likely contributed to a decrease in Earth's surface temperature. Climate scientists are concerned that this effect may have partially offset the global warming caused by the increase in greenhouse gases. Why concerned? In the short term, a reduction in global warming seems a good thing. However, it may be giving us a false sense of security by partially masking the severity of the greenhouse gas induced problem, leading us to take action less quickly than we should to mitigate that problem.
Global dimming may also be interfering with the water cycle. Less sunlight upon water (especially the oceans) leads to a diminished evaporation rate. This may be responsible for droughts in some regions.
There are, of course, natural causes of variation in the amount of sunlight incident upon Earth's surface. Over the course of the 11-year sunspot cycle, the brightness of the Sun varies; but only by about 0.1% in visible wavelengths. Large volcanic eruptions that spew great clouds of ash high into the atmosphere generate effects similar to those caused by some anthropogenic sources of aerosols. However, volcanic ash does not persist in the atmosphere for more than several months to a few years at most. Scientists are aware of major eruptions that have occurred in recent years, and are therefore able to "subtract out" the known effects of these eruptions and of changes in the Sun's brightness over the course of the solar cycle. After this subtraction, the longer-term global dimming trend persists. Or did, up until the 1990s!
More recently, the global dimming effect has apparently reversed. Since the 1990s, instead of continuing to dim, there has actually been a trend towards brightening. Climate scientist believe that more stringent requirements on industry, especially in Europe and North America, have led to significant decreases in the aerosol emissions from these regions. The "Clean Air Act" in the United States and similar policies in Europe have reduced aerosol emissions by roughly 50%. Cleaner air is, of course, a good thing. However, removal of the global dimming effect should increase the amount of sunlight reaching Earth's surface as compared to the recent past. The increase in incident radiation, in combination with a growing greenhouse effect from the continuing emissions of greenhouse gases, may lead to an accelerated rate of global warming.
The effect of aerosols on climate is an area of active research. As is the case with most aspects of climate science, the issues involved are complex. We need to continue to make measurements in the coming years to be certain that the global dimming trend has either disappeared or reversed itself. The effect of aerosols on clouds and climate is not as simple as "more aerosols mean more clouds and greater albedo and hence less light at the surface and thus cooling". Climate scientists must determine what types of clouds are produced at what altitudes by various combinations of aerosols. Some aerosol-induced clouds have especially small cloud droplets, and are thus particularly good at reflecting sunlight. Others have larger droplets and tend to create rain (in certain geographic areas!), which alters the water cycle. Though increased cloudiness blocks incoming sunlight in the day, it also blocks outgoing infrared radiation at night, enhancing the greenhouse effect. Though black carbon prevents sunlight from reaching Earth's surface, the light it absorbs is reradiated as heat into the atmospheric layers in which it is present. Scientists still have much to learn about how alterations in energy flow at various elevations impacts the overall character of those atmospheric layers.