Changing Planet: Ocean Acidification - The Chemistry is Less than Basic!
| Summary: |
Students will use a pH indicator solution to detect the presence of carbon dioxide and changes in acidity, and to model ocean-atmosphere interactions. |
Materials: For each four-student team
|
| Source: |
Adapted by NESTA/Windows to the Universe team members Missy Holzer, Jennifer Bergman, and Roberta Johnson from the Carbon Dioxide Sources and Sinks activity on Windows to the Universe. |
|
| Grade level: |
7-10 |
|
| Time: |
This activity requires careful preparation including some set-up the previous day. It is recommended that the directions be read carefully before beginning this activity.
|
|
|
Student Learning Outcomes:
|
|
|
| Lesson format: |
Laboratory Experiment |
|
| Standards Addressed: |
|
DIRECTIONS:
- For background information on the processes and consequences of ocean acidification on marine ecosystems watch Changing Planet: Ocean Acidification. Also, explore these topics on the Windows to the Universe website at the links listed below. Before starting this lesson, review the pH scale using household acids and bases and pH strips or indicators. This lesson investigates the cycling of carbon in the Earth system, and uses the terms sources and sinks to refer to the sources of carbon/carbon dioxide and sinks as the places that take in carbon/carbon dioxide (the key sink being our oceans).
- Gather materials and print out the student worksheet. In Part 1 of this lesson, students gain experience in detecting CO2 through the BTB reaction by using a pure CO2 gas made from the reaction of baking soda and vinegar. In Part 2 of this lesson, students determine if animals are a source of CO2, and in Parts 3 and Part 4 students determine if plants are a source or a sink of CO2 (or both). Be sure to set up part 3 for students the day before. Part 5 investigates whether fossil fuels are a source of CO2, and in Part 6, students investigate the effect of CO2 on calcifying marine organisms.
- Set up a model to demonstrate the procedure for students. Monitor students as they perform each part of the investigation, and ensure they are making & recording careful observations along the way.
Procedure for Part 5:
- Blow up the balloons and allow the balloons to deflate. This will stretch the rubber and make them easier to fill with the relatively low-pressure exhaust.
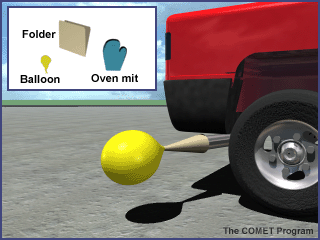
- Prepare a cone to collect the car exhaust by rolling up a manila folder lengthwise. One end must be larger than the opening for the car's tail pipe and the other end must be small enough for the balloon to fit over it.
- Use plenty of tape to hold the cone in shape and to make the sides of the cone fairly airtight. Note: the paper funnel will work for several fillings without burning. DO NOT use a plastic funnel. As the exhaust pipe heats up, the plastic may melt. You may use a metal funnel, but be VERY careful to avoid any skin contact with the hot metal.
- Have an assistant turn on the car (make sure brake is on).
- Put the balloon on the small end of the cone.
- Using heat resistant mitts, approach the exhaust pipe from the side. Place the large end of the cone over the tail pipe. Use the gloved hand to help form a seal between the cone and the exhaust pipe. DO NOT BREATHE THE EXHAUST. The balloon should fill quickly; if not, have your assistant step lightly on the accelerator. See image below for assistance.
- When the balloon is filled, have an assistant use a twist tie or two to tightly seal the balloon. Do this by twisting the neck several times and doubling it over once, then place the twist tie around the constricted area.
- Fill a test tube approximately 1/3 full of BTB.
- Take the exhaust-filled balloon, and carefully untwist the tie while holding the neck of the balloon so that the gas does not escape (don't tie the balloon though).
- While still preventing the gas from escaping, insert a straw into the neck of the balloon up to the twisted portion. Have one team member seal the opening of the balloon tightly to one side, pinching it off with their fingers. You may need to practice this a few times with a regular air-filled balloon.
- Insert the straw into test tube.
- Insert a cotton ball at the top of test tube to help hold the straw steady.
- Gently release air from the balloon by slowly untwisting the neck. Allow the gas to bubble out at a steady rate until the balloon is empty.
Applications
- After students have collected all the data, assist them in applying their results to the issue of ocean acidification. Walk them through a general model of the carbon cycle, and then focus on how carbon dioxide dissolves in oceans and what happens to the carbon dioxide once it is dissolved. For older students, walk them through the chemical equations that are involved in this process.
- Finally, engage students in a discussion about the global implications of an ocean that is more acidic focusing them on marine food chains and the fishing industry. Have students research a variety of organisms that rely on the availability of carbonate ions to create their exoskeletons, and their response to an increase in seawater acidity.
ASSESSMENT:
In this activity, the students have examined several sources of carbon dioxide. Ask them the following questions:
- If you wanted to reduce the rate at which carbon dioxide is increasing in the atmosphere, which source would be most important to control? Explain why.
- Would there be problems with such controls? If so, what might they be?
- Ask students to devise their own experiment to test other sources and sinks of carbon dioxide (e.g. carbonated beverages, lime based chalk, etc.).
LAB SAFETY:
- Automobile exhaust collection important note: Carbon monoxide is an odorless, moderately toxic, poisonous, and flammable gas. It is recommended that this part of the lesson be done as a teacher demonstration, however, in a well ventilated room, students could do this activity. In that case, teachers should provide students with balloons full of car exhaust. It is not recommended that students participate in filling the balloons with car exhaust unless they have an adult assistant (or two).
- Follow all lab safety precautions when working with chemicals and lab ware.
CLEAN-UP:
Dispose of all used materials properly. Clean and store equipment for future use.
EXTENSIONS:
- To introduce this lesson to younger students have them perform the home lab of placing an egg in a glass of vinegar overnight, and report the results to the class. Upon doing this, they will begin to connect the ability of an acid to dissolve shells.
- Students can develop their own experiment using variations of the above procedure to model different marine ecosystems. For instance students can determine the rate of carbon dioxide take-up as a function of the temperature of the water, or students can substitute homemade or real seawater for the distilled water used in this lab.
- Students can investigate real-time concentrations of carbon dioxide in the ocean and atmosphere using data from the Papa mooring located at 145W and 50N.
BACKGROUND INFORMATION:
Increasing amounts of carbon dioxide are released into the atmosphere from burning of fossil fuels. Some of that carbon dioxide makes its way into the world’s oceans. This changes the chemistry of seawater, lowering its pH, making it more acidic, which could have a large impact on marine life in the future.
Marine creatures such as corals, clams, snails, and many types of algae and plankton build their skeletons and shells from calcium carbonate. These creatures get the chemical building blocks they need to form the calcium carbonate mineral of their skeletons from seawater. As seawater gets more acidic with more carbon dioxide dissolved in it, these creatures might have a harder time making their skeletons and shells.
Calcium carbonate mineral dissolves in acid. Try it out for yourself. Put a clam shell (one that you don’t want to keep) into a container of vinegar and wait. Vinegar is an acid. Within a few hours will notice that your clam shell is disappearing. The calcium carbonate that makes up the shell is dissolving into the acidic vinegar.
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at 25 °C (77 °F). Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline.Seawater's pH is not dropping as low as vinegar. Vinegar has a pH of about 3. The pH of seawater varies between 7 and 8, so it is a little more basic than neutral. Since the start of the Industrial Revolution, pH of seawater has dropped about 0.1. In the next century it is expected to drop another 0.1-0.35. But scientists suspect that even these small changes can make a big difference to the creatures that need to build their shells.
Because reef corals build massive structures from calcium carbonate, and because those structures become a home to diverse communities of marine life, the impact of increasing acidification on corals is of particular interest to many scientists.
The current research indicated that for a doubling of the partial pressure of carbon dioxide (pCO2), rates of calcification decreased in corals an average of 30%. The rate is affected by many other factors besides the concentration of CO2 dissolved in the water. The temperature, light, and nutrients all affect calcification rates too.
Lower rates of calcification will likely impact marine food webs, possibly changing the biodiversity of the ocean.
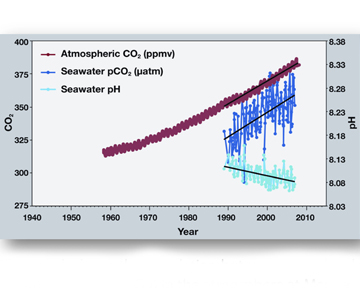
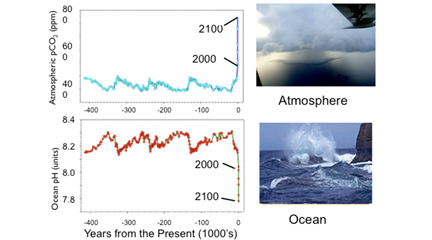
Sometimes a picture is worth a thousand words. This image (which students are asked to analyze in the student worksheet) shows the correlation between rising levels of carbon dioxide (CO2) in the atmosphere at Mauna Loa with rising CO2 levels in the nearby ocean at Station Aloha. As more CO2 accumulates in the ocean, the PH of the ocean decreases. (Source: NOAA PMEL Carbon Program)Another helpful image to go over with your students is the following comparative graph set. The top graph shows the variation of CO2 in the Earth's atmosphere over the last 400,000 years. You can see the blue line goes up and down with natural variations in levels of CO2 until just before Present where CO2 levels spike drastically upwards due to human contribution of the gas. Projected levels of CO2 are shown for 2100. On the bottom graph, the ocean's pH is shown. You can see that this inversely varies with the atmospheric CO2. You can see natural variations of the ocean's pH until just before Present, where the pH plummets from natural levels. Projected pH level is around 7.8 in 2100, much lower (more acidic) that the ocean is now.
RELATED SECTIONS OF THE WINDOWS TO THE UNIVERSE WEBSITE:
- Effects of Climate Change Today
- What Is Climate?
- Modeling the Future of Climate Change
- Climate and Global Change
- The Carbon Cycle
- Ocean Chemistry
- Carbon Dioxide - CO2
- Changing Planet: Fading Corals
- Ocean Life
OTHER RESOURCES:
- Oceanography - A Special Issue on Ocean Acidification
- Virtual Urchin: Acid Ocean from Stanford University
- Acid Test: The Global Challenge of Ocean Acidification
- Ocean Acidification Demonstrations
- NOAA Ocean Acidification Factsheet
- Home Science Tools for Inexpensive Coral and Shell Samples