Click on image for full size
Images courtesy COMET.
Atmospheric Chemistry
Chemistry plays an influential role in the behavior of Earth's atmosphere. The various gases in the atmosphere are constantly mixing with and reacting with each other. Gases released by the oceans, emitted by plants, animals, and microbes, and poured into the atmosphere by human activities continually alter the balance of atmospheric chemistry.
The activities of humans play an increasingly important role in atmospheric chemistry. Fossil fuel burning generates sulfur oxides, which create sulfuric acid - a component of acid rain. Exhaust gases from cars and trucks produce nitrogen oxides, which contribute to the formation of smog and of nitric acid - another component of acid rain.
Nature also does its share to alter the chemistry of the atmosphere. Volcanic eruptions, wildfires, lightning, and UV radiation from the Sun all add materials to the atmosphere or chemically alter those already there. The oceans and the biosphere exchange vast quantities of gases with the atmosphere. The Carbon and Nitrogen Cycles play key roles in these processes.
You might also be interested in:
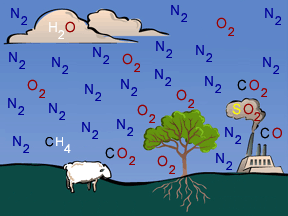
Earth's atmosphere consists of about 78% nitrogen, 20% oxygen, and a mixture of small amounts of numerous other ingredients. Some of the minor constituents do, however, have big impacts. For example, greenhouse
...more
Air pollution comes from many different sources. Natural processes that affect air quality include volcanic activity, which produce sulfur, chlorine, and ash particulates, and wildfires, which produce
...more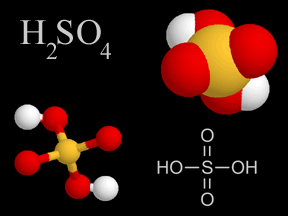
Sulfuric acid is a viscous, oily liquid and a strong acid which can cause severe burns. Sulfuric acid consists of sulfur, oxygen, and hydrogen atoms. Sulfuric acid is one of the components of acid rain.
...more
Acid rain is a general term used to describe different kinds of acidic air pollution. Although some acidic air pollutants return directly back to Earth, a lot of it returns in rain, snow, sleet, hail,
...more
Smog is a type of air pollution. Smog is a mixture of smoke and fog, hence the name (SMoke + fOG = SMOG). Victorian-era London was famous for its thick smogs, which resulted from the city's frequent, naturally
...more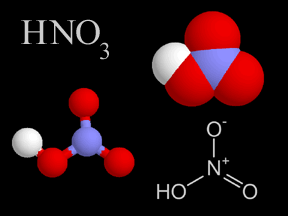
Nitric acid is a colorless, corrosive liquid and a toxic acid which can cause severe burns. Nitric acid consists of nitrogen, oxygen, and hydrogen atoms. Nitric acid, in its gas phase, is present in very
...more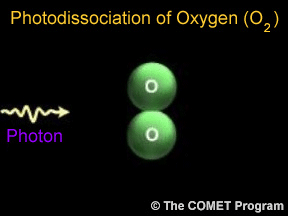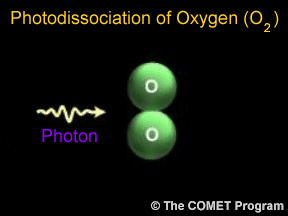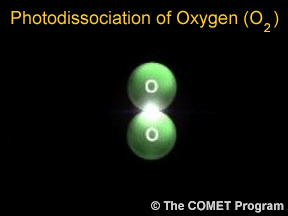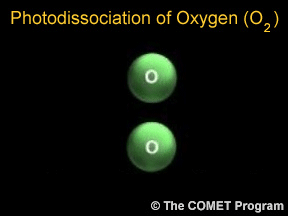
Sometimes when a photon hits a molecule, the energy from the photon causes the molecule to break apart. Scientists use the term "photodissociation" for such events. Photodissociation plays a very important
...more














