ExploraTour: A Peek into the Lives of the Stars
Why does nuclear fusion in stars stop after an iron core is created?
The diagram at the left gives the answer to that question. It shows the excess energy per nucleon plotted against the atomic mass number of an element.
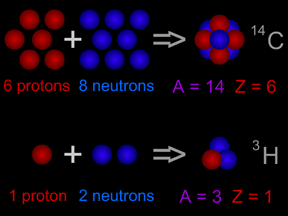
To illustrate what that means let's consider one particular example. The carbon nucleus (atomic mass number of 12) is made up of 6 protons and 6 neutrons. If we add up the mass of these particles, the carbon nucleus should have a mass of 12.098940. Yet the actual mass of the carbon nucleus is only 12.00000. There is a difference in the mass of 0.098940.
This mass was converted into energy and released when the carbon nucleus was created. If we divide this energy by the number of nucleons (protons and neutrons), we get the quantity plotted on the vertical axis in the figure.
Less and less energy is released as heavier and heavier elements (moving toward Iron-56) are created by fusing lighter elements together. In fact, when we attempt to fuse iron to produce heavier elements, no energy is released. Energy must be added.
When a star's core is converted to iron, nuclear fusion is no longer an energy source. With nothing to oppose gravity, the core collapses with blinding speed.